Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence
- PMID: 31000627
- PMCID: PMC6552422
- DOI: 10.1074/jbc.RA118.006615
Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence
Abstract
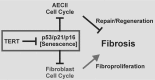
Mutations in the genes encoding telomerase reverse transcriptase (TERT) and telomerase's RNA components as well as shortened telomeres are risk factors for idiopathic pulmonary fibrosis, where repetitive injury to the alveolar epithelium is considered a key factor in pathogenesis. Given the importance of TERT in stem cells, we hypothesized that TERT plays an important role in epithelial repair and that its deficiency results in exacerbation of fibrosis by impairing this repair/regenerative process. To evaluate the role of TERT in epithelial cells, we generated type II alveolar epithelial cell (AECII)-specific TERT conditional knockout (SPC-Tert cKO) mice by crossing floxed Tert mice with inducible SPC-driven Cre mice. SPC-Tert cKO mice did not develop pulmonary fibrosis spontaneously up to 9 months of TERT deficiency. However, upon bleomycin treatment, they exhibited enhanced lung injury, inflammation, and fibrosis compared with control mice, accompanied by increased pro-fibrogenic cytokine expression but without a significant effect on AECII telomere length. Moreover, selective TERT deficiency in AECII diminished their proliferation and induced cellular senescence. These findings suggest that AECII-specific TERT deficiency enhances pulmonary fibrosis by heightening susceptibility to bleomycin-induced epithelial injury and diminishing epithelial regenerative capacity because of increased cellular senescence. We confirmed evidence for increased AECII senescence in idiopathic pulmonary fibrosis lungs, suggesting potential clinical relevance of the findings from our animal model. Our results suggest that TERT has a protective role in AECII, unlike its pro-fibrotic activity, observed previously in fibroblasts, indicating that TERT's role in pulmonary fibrosis is cell type-specific.
Keywords: epithelial cell; fibrosis; idiopathic pulmonary fibrosis (IPF); inflammation; lung disease; pulmonary dysfunction; senescence; telomerase; telomerase reverse transcriptase (TERT).
© 2019 Liu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures



Similar articles
-
Telomerase deficiency does not alter bleomycin-induced fibrosis in mice.Exp Lung Res. 2012 Apr;38(3):124-34. doi: 10.3109/01902148.2012.658148. Exp Lung Res. 2012. PMID: 22394286 Free PMC article.
-
Telomerase and telomere length in pulmonary fibrosis.Am J Respir Cell Mol Biol. 2013 Aug;49(2):260-8. doi: 10.1165/rcmb.2012-0514OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526226 Free PMC article. Clinical Trial.
-
Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs.J Biol Chem. 2015 Dec 25;290(52):30813-29. doi: 10.1074/jbc.M115.681619. Epub 2015 Oct 30. J Biol Chem. 2015. PMID: 26518879 Free PMC article.
-
Molecular Mechanisms of Alveolar Epithelial Stem Cell Senescence and Senescence-Associated Differentiation Disorders in Pulmonary Fibrosis.Cells. 2022 Mar 3;11(5):877. doi: 10.3390/cells11050877. Cells. 2022. PMID: 35269498 Free PMC article. Review.
-
Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis.Dis Model Mech. 2013 Jan;6(1):9-17. doi: 10.1242/dmm.010736. Dis Model Mech. 2013. PMID: 23268535 Free PMC article. Review.
Cited by
-
Canonical and extra-telomeric functions of telomerase: Implications for healthy ageing conferred by endurance training.Aging Cell. 2023 Jun;22(6):e13836. doi: 10.1111/acel.13836. Epub 2023 Apr 11. Aging Cell. 2023. PMID: 37041671 Free PMC article. Review.
-
Is there a role for specialized pro-resolving mediators in pulmonary fibrosis?Pharmacol Ther. 2023 Jul;247:108460. doi: 10.1016/j.pharmthera.2023.108460. Epub 2023 May 26. Pharmacol Ther. 2023. PMID: 37244406 Free PMC article. Review.
-
New Insights via RNA Profiling of Formalin-Fixed Paraffin-Embedded Lung Tissue of Pulmonary Fibrosis Patients.Int J Mol Sci. 2023 Nov 25;24(23):16748. doi: 10.3390/ijms242316748. Int J Mol Sci. 2023. PMID: 38069069 Free PMC article.
-
Fibroblast Senescence in Idiopathic Pulmonary Fibrosis.Front Cell Dev Biol. 2020 Nov 25;8:593283. doi: 10.3389/fcell.2020.593283. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33324646 Free PMC article. Review.
-
Cellular Senescence: A Troy Horse in Pulmonary Fibrosis.Int J Mol Sci. 2023 Nov 16;24(22):16410. doi: 10.3390/ijms242216410. Int J Mol Sci. 2023. PMID: 38003600 Free PMC article. Review.
References
-
- Alder J. K., Chen J. J., Lancaster L., Danoff S., Su S. C., Cogan J. D., Vulto I., Xie M., Qi X., Tuder R. M., Phillips J. A. 3rd, Lansdorp P. M., Loyd J. E., and Armanios M. Y. (2008) Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl. Acad. Sci. U.S.A. 105, 13051–13056 10.1073/pnas.0804280105 - DOI - PMC - PubMed
-
- Armanios M. Y., Chen J. J., Cogan J. D., Alder J. K., Ingersoll R. G., Markin C., Lawson W. E., Xie M., Vulto I., Phillips J. A. 3rd, Lansdorp P. M., Greider C. W., and Loyd J. E. (2007) Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 10.1056/NEJMoa066157 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

