Prokinetic effects of spinal cord stimulation and its autonomic mechanisms in dogs
- PMID: 30983068
- PMCID: PMC6996459
- DOI: 10.1111/nmo.13596
Prokinetic effects of spinal cord stimulation and its autonomic mechanisms in dogs
Abstract
Background: Spinal cord stimulation (SCS) is widely used to treat chronic pain by inhibiting sympathetic activity; however, it is unknown whether it exerts a prokinetic effect on gastric motility. Our aim was to explore effects and possible mechanisms of SCS on glucagon-induced gastric dysmotility and dysrhythmia.
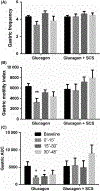
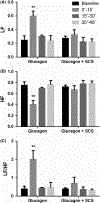
Methods: Seven female dogs with electrodes chronically placed on the dorsal column of the spinal cord between T10 and T12 segments were studied in 2 randomized sessions (glucagon + sham-SCS, glucagon + SCS). SCS at T10 using a set of optimized stimulation parameters was performed for 30 minute immediately after glucagon injection. The antral manometry, electrogastrogram, and electrocardiogram were recorded to assess gastric contractions, gastric slow waves (GSW), and autonomic functions, respectively.
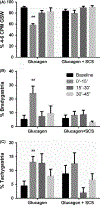
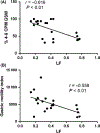
Key results: (a) Compared to baseline, glucagon decreased antral motility index (MI) (6315 ± 565 vs 3243 ± 775, P < 0.001), reduced the percentage of normal GSW (89 ± 3% vs 58 ± 3%, P < 0.01), and increased sympathetic activity (0.25 ± 0 0.06 vs 0.60 ± 0.07, P < 0.01). (b) The sympathetic activity was negatively correlated with antral MI (r = -0.558; P < 0.01) and the percentage of gastric normal slow wave (r = -0.616; P < 0.01). (c) SCS prevented the glucagon-induced impairment in antral hypomotility (MI: 5770 ± 927 vs 5521 ± 1238, P > 0.05) and GSW abnormalities (% of normal waves: 84 ± 4% vs 79 ± 6%, P > 0.05) and sympathetic activity (0.27 ± 0.03 vs 0.33 ± 0.07, P > 0.05).
Conclusion: Spinal cord stimulation dramatically improves glucagon-induced impairment in gastric contractions and slow waves by inhibiting sympathetic activity.
Keywords: autonomic function; gastric motility; gastric slow waves; neuromodulation; spinal cord stimulation.
© 2019 John Wiley & Sons Ltd.
Conflict of interest statement
DISCLOSURE
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Therapeutic potential of spinal cord stimulation for gastrointestinal motility disorders: a preliminary rodent study.Neurogastroenterol Motil. 2014 Mar;26(3):377-84. doi: 10.1111/nmo.12273. Epub 2013 Dec 17. Neurogastroenterol Motil. 2014. PMID: 24341686
-
A Novel Approach in Spinal Cord Stimulation for Enhancing Gastric Motility: A Preliminary Study on Canines.J Neurogastroenterol Motil. 2020 Jan 30;26(1):147-159. doi: 10.5056/jnm19101. J Neurogastroenterol Motil. 2020. PMID: 31917917 Free PMC article.
-
Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs.Am J Physiol Gastrointest Liver Physiol. 2008 Sep;295(3):G614-20. doi: 10.1152/ajpgi.90322.2008. Epub 2008 Jul 24. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18653722
-
Electroacupuncture via chronically implanted electrodes improves gastric dysmotility mediated by autonomic-cholinergic mechanisms in a rodent model of functional dyspepsia.Neurogastroenterol Motil. 2018 Oct;30(10):e13381. doi: 10.1111/nmo.13381. Epub 2018 Jun 1. Neurogastroenterol Motil. 2018. PMID: 29856090
-
A Comprehensive Outcome-Specific Review of the Use of Spinal Cord Stimulation for Complex Regional Pain Syndrome.Pain Pract. 2017 Apr;17(4):533-545. doi: 10.1111/papr.12513. Epub 2016 Oct 14. Pain Pract. 2017. PMID: 27739179 Review.
References
-
- Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081. - PubMed
-
- Venara A, Neunlist M, Slim K, et al. Postoperative ileus: Pathophysiology, incidence, and prevention. J Vise Surg. 2016;153:439–446. - PubMed
-
- Sato T, Kitahara F, Nakamura T, Kojima Y, Fujino MA. [Peptic ulcer in patients with diabetes mellitus]. Nihon Rinsho. 2002;60:1580–1584. - PubMed
-
- van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. Generation and propagation of gastric slow waves. Clin Exp Pharmacol Physiol. 2010;37:516–524. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

