Context-dependent EMT programs in cancer metastasis
- PMID: 30975895
- PMCID: PMC6504222
- DOI: 10.1084/jem.20181827
Context-dependent EMT programs in cancer metastasis
Abstract
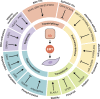
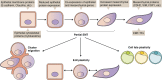
Epithelial-mesenchymal transition (EMT) is a developmental process whereby stationary, adherent cells acquire the ability to migrate. EMT is critical for dramatic cellular movements during embryogenesis; however, tumor cells can reactivate EMT programs, which increases their aggressiveness. In addition to motility, EMT is associated with enhanced stem cell properties and drug resistance; thus it can drive metastasis, tumor recurrence, and therapy resistance in the context of cancer. However, the precise requirements for EMT in metastasis have not been fully delineated, with different tumor types relying on discrete EMT effectors. Most tumor cells do not undergo a full EMT, but rather adopt some qualities of mesenchymal cells and maintain some epithelial characteristics. Emerging evidence suggests that partial EMT can drive distinct migratory properties and enhance the epithelial-mesenchymal plasticity of cancer cells as well as cell fate plasticity. This review discusses the diverse regulatory mechanisms and functional consequences of EMT, with an emphasis on the importance of partial EMT.
© 2019 Aiello and Kang.
Figures
Similar articles
-
Stability of the hybrid epithelial/mesenchymal phenotype.Oncotarget. 2016 May 10;7(19):27067-84. doi: 10.18632/oncotarget.8166. Oncotarget. 2016. PMID: 27008704 Free PMC article.
-
A Spectrum of Cell States During the Epithelial-to-Mesenchymal Transition.Methods Mol Biol. 2021;2179:3-6. doi: 10.1007/978-1-0716-0779-4_1. Methods Mol Biol. 2021. PMID: 32939707
-
Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas.Pharmacol Ther. 2019 Feb;194:161-184. doi: 10.1016/j.pharmthera.2018.09.007. Epub 2018 Sep 28. Pharmacol Ther. 2019. PMID: 30268772 Review.
-
OVOL2 links stemness and metastasis via fine-tuning epithelial-mesenchymal transition in nasopharyngeal carcinoma.Theranostics. 2018 Mar 8;8(8):2202-2216. doi: 10.7150/thno.24003. eCollection 2018. Theranostics. 2018. PMID: 29721073 Free PMC article.
-
Epithelial-mesenchymal transition in tumor metastasis.Mol Oncol. 2017 Jan;11(1):28-39. doi: 10.1002/1878-0261.12017. Epub 2016 Dec 9. Mol Oncol. 2017. PMID: 28085222 Free PMC article. Review.
Cited by
-
CircCSPP1 Functions as a ceRNA to Promote Colorectal Carcinoma Cell EMT and Liver Metastasis by Upregulating COL1A1.Front Oncol. 2020 Jun 16;10:850. doi: 10.3389/fonc.2020.00850. eCollection 2020. Front Oncol. 2020. PMID: 32612946 Free PMC article.
-
Identification and validation of metastasis-related gene ZG16 in the prognosis and progression in colorectal cancer.Front Oncol. 2024 Jul 24;14:1409329. doi: 10.3389/fonc.2024.1409329. eCollection 2024. Front Oncol. 2024. PMID: 39114307 Free PMC article.
-
lncCRLA Enhanced Chemoresistance in Lung Adenocarcinoma That Underwent EpithelialMesenchymal Transition.Oncol Res. 2022 Jan 31;28(9):857-872. doi: 10.3727/096504021X16203818567367. Epub 2021 May 13. Oncol Res. 2022. PMID: 33985619 Free PMC article.
-
The Cancer Cell Dissemination Machinery as an Immunosuppressive Niche: A New Obstacle Towards the Era of Cancer Immunotherapy.Front Immunol. 2021 Apr 13;12:654877. doi: 10.3389/fimmu.2021.654877. eCollection 2021. Front Immunol. 2021. PMID: 33927723 Free PMC article. Review.
-
Long non‑coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR‑1321 and regulating tight junction protein 3 expression.Mol Med Rep. 2020 Oct;22(4):3429-3439. doi: 10.3892/mmr.2020.11428. Epub 2020 Aug 11. Mol Med Rep. 2020. PMID: 32945443 Free PMC article.
References
-
- Aghdassi A., Sendler M., Guenther A., Mayerle J., Behn C.O., Heidecke C.D., Friess H., Büchler M., Evert M., Lerch M.M., and Weiss F.U.. 2012. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut. 61:439–448. 10.1136/gutjnl-2011-300060 - DOI - PubMed
-
- Aiello N.M., Bajor D.L., Norgard R.J., Sahmoud A., Bhagwat N., Pham M.N., Cornish T.C., Iacobuzio-Donahue C.A., Vonderheide R.H., and Stanger B.Z.. 2016. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat. Commun. 7:12819 10.1038/ncomms12819 - DOI - PMC - PubMed
-
- Akalay I., Janji B., Hasmim M., Noman M.Z., André F., De Cremoux P., Bertheau P., Badoual C., Vielh P., Larsen A.K., et al. . 2013. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 73:2418–2427. 10.1158/0008-5472.CAN-12-2432 - DOI - PubMed

