High Glucose Concentrations Negatively Regulate the IGF1R/Src/ERK Axis through the MicroRNA-9 in Colorectal Cancer
- PMID: 30965609
- PMCID: PMC6523516
- DOI: 10.3390/cells8040326
High Glucose Concentrations Negatively Regulate the IGF1R/Src/ERK Axis through the MicroRNA-9 in Colorectal Cancer
Abstract
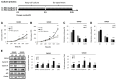
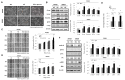
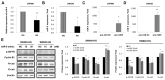
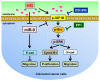
Studies have revealed that people with hyperglycemia have a high risk of colorectal cancer (CRC). Hyperglycemia may be responsible for supplying energy to CRC cells. However, the potential molecular mechanism for this association remains unclear. Furthermore, microRNA-9 (miR-9) has a tumor-suppressive function in CRC. Aberrant reduced expression of miR-9 is involved in the development and progression of malignancy caused by a high glucose (HG) concentration. In this study, we used an HG concentration to activate miR-9 downregulation in CRC cells. Our results indicated that miR-9 decreased the insulin-like growth factor-1 receptor (IGF1R)/Src signaling pathway and downstream cyclin B1 and N-cadherin but upregulated E-cadherin. The HG concentration not only promoted cell proliferation, increased the G1 population, and modulated epithelial-to-mesenchymal transition (EMT) protein expression and morphology but also promoted the cell migration and invasion ability of SW480 (low metastatic potential) and SW620 (high metastatic potential) cells. In addition, low glucose concentrations could reverse the effect of the HG concentration in SW480 and SW620 cells. In conclusion, our results provide new evidence for multiple signaling pathways being regulated through hyperglycemia in CRC. We propose that blood sugar control may serve as a potential strategy for the clinical management of CRC.
Keywords: Src; colorectal cancer; epithelial to mesenchymal transition; extracellular signal-regulated kinase (ERK1/2); high concentration of glucose; insulin-like growth factor-1 receptor; metastasis; microRNA-9; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Regulation of Src Family Kinases during Colorectal Cancer Development and Its Clinical Implications.Cancers (Basel). 2020 May 23;12(5):1339. doi: 10.3390/cancers12051339. Cancers (Basel). 2020. PMID: 32456226 Free PMC article. Review.
-
MicroRNA-141-3p affected proliferation, chemosensitivity, migration and invasion of colorectal cancer cells by targeting EGFR.Int J Biochem Cell Biol. 2020 Jan;118:105643. doi: 10.1016/j.biocel.2019.105643. Epub 2019 Nov 5. Int J Biochem Cell Biol. 2020. PMID: 31704502
-
miR-196a-5p promotes metastasis of colorectal cancer via targeting IκBα.BMC Cancer. 2019 Jan 8;19(1):30. doi: 10.1186/s12885-018-5245-1. BMC Cancer. 2019. PMID: 30621631 Free PMC article.
-
Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article.
-
Lessons to Learn for Adequate Targeted Therapy Development in Metastatic Colorectal Cancer Patients.Int J Mol Sci. 2021 May 9;22(9):5019. doi: 10.3390/ijms22095019. Int J Mol Sci. 2021. PMID: 34065119 Free PMC article. Review.
Cited by
-
Regulation of Src Family Kinases during Colorectal Cancer Development and Its Clinical Implications.Cancers (Basel). 2020 May 23;12(5):1339. doi: 10.3390/cancers12051339. Cancers (Basel). 2020. PMID: 32456226 Free PMC article. Review.
-
FLT3-TKD in the prognosis of patients with acute myeloid leukemia: A meta-analysis.Front Oncol. 2023 Feb 17;13:1086846. doi: 10.3389/fonc.2023.1086846. eCollection 2023. Front Oncol. 2023. PMID: 36874106 Free PMC article.
-
High glucose inhibits autophagy and promotes the proliferation and metastasis of colorectal cancer through the PI3K/AKT/mTOR pathway.Cancer Med. 2024 Jun;13(11):e7382. doi: 10.1002/cam4.7382. Cancer Med. 2024. PMID: 38872380 Free PMC article.
-
Systematic Elucidation of the Mechanism of Quercetin against Gastric Cancer via Network Pharmacology Approach.Biomed Res Int. 2020 Sep 3;2020:3860213. doi: 10.1155/2020/3860213. eCollection 2020. Biomed Res Int. 2020. PMID: 32964029 Free PMC article.
-
The Burden of Early-Onset Colorectal Cancer and Its Risk Factors from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019.Cancers (Basel). 2022 Jul 19;14(14):3502. doi: 10.3390/cancers14143502. Cancers (Basel). 2022. PMID: 35884567 Free PMC article.
References
-
- Lee M.Y., Lin K.D., Hsiao P.J., Shin S.J. Thiamine corrects delayed replication and decreases production of lactate and advanced glycation end-products in bovine retinal and human umbilical vein endothelial cells cultured under high glucose conditions. Metabolism. 2012;61:242–249. doi: 10.1016/j.metabol.2011.06.020. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

