Expression profiling of immune inhibitory Siglecs and their ligands in patients with glioma
- PMID: 30953118
- PMCID: PMC6529385
- DOI: 10.1007/s00262-019-02332-w
Expression profiling of immune inhibitory Siglecs and their ligands in patients with glioma
Abstract
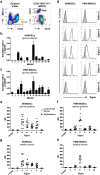
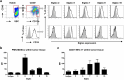
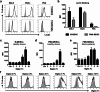
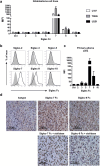
Gliomas appear to be highly immunosuppressive tumors, with a strong myeloid component. This includes MDSCs, which are a heterogeneous, immature myeloid cell population expressing myeloid markers Siglec-3 (CD33) and CD11b and lacking markers of mature myeloid cells including MHC II. Siglec-3 is a member of the sialic acid-binding immunoglobulin-like lectin (Siglec) family and has been suggested to promote MDSC expansion and suppression. Siglecs form a recently defined family of receptors with potential immunoregulatory functions but only limited insight in their expression on immune regulatory cell subsets, prompting us to investigate Siglec expression on MDSCs. We determined the expression of different Siglec family members on monocytic-MDSCs (M-MDSCs) and polymorphnuclear-MDSCs (PMN-MDSCs) from blood of glioma patients and healthy donors, as well as from patient-derived tumor material. Furthermore, we investigated the presence of sialic acid ligands for these Siglecs on MDSCs and in the glioma tumor microenvironment. Both MDSC subsets express Siglec-3, -5, -7 and -9, with higher levels of Siglec-3, -7 and -9 on M-MDSCs and higher Siglec-5 levels on PMN-MDSCs. Similar Siglec expression profiles were found on MDSCs from healthy donors. Furthermore, the presence of Siglec-5 and -9 was also confirmed on PMN-MDSCs from glioma tissue. Interestingly, freshly isolated glioma cells predominantly expressed sialic acid ligands for Siglec-7 and -9, which was confirmed in situ. In conclusion, our data show a distinct Siglec expression profile for M- and PMN-MDSCs and propose possible sialic acid-Siglec interactions between glioma cells and MDSCs in the tumor microenvironment.
Keywords: Glioma; Myeloid-derived suppressor cells; Sialic acids; Siglecs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Regulation of prognosis-related Siglecs in the glioma microenvironment.J Cancer Res Clin Oncol. 2021 Nov;147(11):3343-3357. doi: 10.1007/s00432-021-03762-9. Epub 2021 Sep 1. J Cancer Res Clin Oncol. 2021. PMID: 34472004
-
Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins.Dev Comp Immunol. 2018 Sep;86:219-231. doi: 10.1016/j.dci.2018.05.008. Epub 2018 May 8. Dev Comp Immunol. 2018. PMID: 29751010 Review.
-
Engagement of sialylated glycans with Siglec receptors on suppressive myeloid cells inhibits anticancer immunity via CCL2.Cell Mol Immunol. 2024 May;21(5):495-509. doi: 10.1038/s41423-024-01142-0. Epub 2024 Mar 6. Cell Mol Immunol. 2024. PMID: 38448555 Free PMC article.
-
Human brain sialoglycan ligand for CD33, a microglial inhibitory Siglec implicated in Alzheimer's disease.J Biol Chem. 2022 Jun;298(6):101960. doi: 10.1016/j.jbc.2022.101960. Epub 2022 Apr 20. J Biol Chem. 2022. PMID: 35452678 Free PMC article.
-
Discovery, classification, evolution and diversity of Siglecs.Mol Aspects Med. 2023 Apr;90:101117. doi: 10.1016/j.mam.2022.101117. Epub 2022 Aug 18. Mol Aspects Med. 2023. PMID: 35989204 Free PMC article. Review.
Cited by
-
Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments.Int J Mol Sci. 2021 May 28;22(11):5774. doi: 10.3390/ijms22115774. Int J Mol Sci. 2021. PMID: 34071314 Free PMC article. Review.
-
Regulation of prognosis-related Siglecs in the glioma microenvironment.J Cancer Res Clin Oncol. 2021 Nov;147(11):3343-3357. doi: 10.1007/s00432-021-03762-9. Epub 2021 Sep 1. J Cancer Res Clin Oncol. 2021. PMID: 34472004
-
Enhancing CAR T function with the engineered secretion of C. perfringens neuraminidase.Mol Ther. 2022 Mar 2;30(3):1201-1214. doi: 10.1016/j.ymthe.2021.11.014. Epub 2021 Nov 20. Mol Ther. 2022. PMID: 34813961 Free PMC article.
-
Glycomaterials to Investigate the Functional Role of Aberrant Glycosylation in Glioblastoma.Adv Healthc Mater. 2022 Feb;11(4):e2101956. doi: 10.1002/adhm.202101956. Epub 2021 Dec 29. Adv Healthc Mater. 2022. PMID: 34878733 Free PMC article. Review.
-
Dynamic change in Siglec-15 expression in peritumoral macrophages confers an immunosuppressive microenvironment and poor outcome in glioma.Front Immunol. 2023 May 10;14:1159085. doi: 10.3389/fimmu.2023.1159085. eCollection 2023. Front Immunol. 2023. PMID: 37234161 Free PMC article.
References
-
- Gielen PR. The immunosuppressive network in patients with glioma; focus on the role of myeloid derived suppressor cells (doctoral thesis) Nijmegen: Radboud umc; 2016.
-
- Gousias K, Markou M, Arzoglou V, Voulgaris S, Vartholomatos G, Kostoula A, Voulgari P, Polyzoidis K, Kyritsis AP. Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. J Neuroimmunol. 2010;226(1–2):136–142. doi: 10.1016/j.jneuroim.2010.05.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

