Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4+ T cell activation and HIV-1 latency reversal
- PMID: 30944862
- PMCID: PMC6436934
- DOI: 10.1126/sciadv.aav6322
Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4+ T cell activation and HIV-1 latency reversal
Abstract
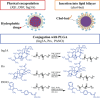
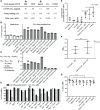
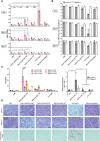
A proposed strategy to cure HIV uses latency-reversing agents (LRAs) to reactivate latent proviruses for purging HIV reservoirs. A variety of LRAs have been identified, but none has yet proven effective in reducing the reservoir size in vivo. Nanocarriers could address some major challenges by improving drug solubility and safety, providing sustained drug release, and simultaneously delivering multiple drugs to target tissues and cells. Here, we formulated hybrid nanocarriers that incorporate physicochemically diverse LRAs and target lymphatic CD4+ T cells. We identified one LRA combination that displayed synergistic latency reversal and low cytotoxicity in a cell model of HIV and in CD4+ T cells from virologically suppressed patients. Furthermore, our targeted nanocarriers selectively activated CD4+ T cells in nonhuman primate peripheral blood mononuclear cells as well as in murine lymph nodes, and substantially reduced local toxicity. This nanocarrier platform may enable new solutions for delivering anti-HIV agents for an HIV cure.
Figures


Similar articles
-
TLR1/2 Agonist Enhances Reversal of HIV-1 Latency and Promotes NK Cell-Induced Suppression of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2021 Aug 10;95(17):e0081621. doi: 10.1128/JVI.00816-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133900 Free PMC article.
-
Latency reversal agents affect differently the latent reservoir present in distinct CD4+ T subpopulations.PLoS Pathog. 2019 Aug 19;15(8):e1007991. doi: 10.1371/journal.ppat.1007991. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31425551 Free PMC article.
-
Anti-HIV-1 ADCC and HIV-1 Env Can Be Partners in Reducing Latent HIV Reservoir.Front Immunol. 2021 Apr 30;12:663919. doi: 10.3389/fimmu.2021.663919. eCollection 2021. Front Immunol. 2021. PMID: 33995393 Free PMC article.
-
The role of latency reversal agents in the cure of HIV: A review of current data.Immunol Lett. 2018 Apr;196:135-139. doi: 10.1016/j.imlet.2018.02.004. Epub 2018 Feb 7. Immunol Lett. 2018. PMID: 29427743 Review.
-
Which therapeutic strategy will achieve a cure for HIV-1?Curr Opin Virol. 2016 Jun;18:14-9. doi: 10.1016/j.coviro.2016.02.001. Epub 2016 Mar 15. Curr Opin Virol. 2016. PMID: 26985878 Review.
Cited by
-
Targeted modulation of immune cells and tissues using engineered biomaterials.Nat Rev Bioeng. 2023 Feb;1(2):107-124. doi: 10.1038/s44222-022-00016-2. Epub 2023 Jan 30. Nat Rev Bioeng. 2023. PMID: 37772035 Free PMC article.
-
The role of latency reversal in HIV cure strategies.J Med Primatol. 2022 Oct;51(5):278-283. doi: 10.1111/jmp.12613. Epub 2022 Aug 27. J Med Primatol. 2022. PMID: 36029233 Free PMC article. Review.
-
Heme Oxygenase 1-Targeted Hybrid Nanoparticle for Chemo- and Immuno-Combination Therapy in Acute Myelogenous Leukemia.Adv Sci (Weinh). 2020 Jun 3;7(13):2000487. doi: 10.1002/advs.202000487. eCollection 2020 Jul. Adv Sci (Weinh). 2020. PMID: 32670766 Free PMC article.
-
Nanotechnology for virus treatment.Nano Today. 2021 Feb;36:101031. doi: 10.1016/j.nantod.2020.101031. Epub 2020 Dec 1. Nano Today. 2021. PMID: 33519948 Free PMC article. Review.
-
Nanoparticle-Based Immunoengineered Approaches for Combating HIV.Front Immunol. 2020 Apr 28;11:789. doi: 10.3389/fimmu.2020.00789. eCollection 2020. Front Immunol. 2020. PMID: 32425949 Free PMC article. Review.
References
-
- Lorenzo-Redondo R., Fryer H. R., Bedford T., Kim E.-Y., Archer J., Kosakovsky Pond S. L., Chung Y.-S., Penugonda S., Chipman J. G., Fletcher C. V., Schacker T. W., Malim M. H., Rambaut A., Haase A. T., McLean A. R., Wolinsky S. M., Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530, 51–56 (2016). - PMC - PubMed
-
- Fletcher C. V., Staskus K., Wietgrefe S. W., Rothenberger M., Reilly C., Chipman J. G., Beilman G. J., Khoruts A., Thorkelson A., Schmidt T. E., Anderson J., Perkey K., Stevenson M., Perelson A. S., Douek D. C., Haase A. T., Schacker T. W., Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. U.S.A. 111, 2307–2312 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

