Glucosamine inhibits IL-1β expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome
- PMID: 30944389
- PMCID: PMC6447579
- DOI: 10.1038/s41598-019-42130-z
Glucosamine inhibits IL-1β expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome
Abstract
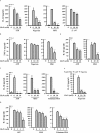
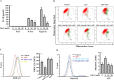
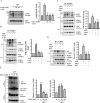
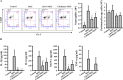
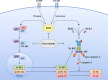
The NLRP3 inflammasome promotes the pathogenesis of metabolic, neurodegenerative and infectious diseases. Increasing evidences show that the NLRP3 inflammasome is a promising therapeutic target in inflammatory diseases. Glucosamine is widely used as a dietary supplement to promote the health of cartilage tissue and is expected to exert anti-inflammatory activity in joint inflammation, which is a nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome-associated complication. Here, we investigated whether GlcN inhibits the NLRP3 inflammasome and dissected the underlying molecular mechanisms. We found that GlcN suppressed the NLRP3 inflammasome in mouse and human macrophages. A mechanistic study revealed that GlcN inhibited the expression of NLRP3 and IL-1β precursor by reducing reactive oxygen species generation and NF-κB activation in lipopolysaccharide-activated macrophages. GlcN also suppressed mitochondrial reactive oxygen species generation and mitochondrial integrity loss in NLRP3-activated macrophages. Additionally, GlcN disrupted NLRP3 inflammasome assembly by inhibiting NLRP3 binding to PKR, NEK7 and ASC. Furthermore, oral administration of GlcN reduced peritoneal neutrophils influx and lavage fluids concentrations of IL-1β, IL-6 MCP-1 and TNF-α in uric acid crystal-injected mice. These results indicated that GlcN might be a novel dietary supplement for the amelioration of NLRP3 inflammasome-associated complications.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
1'-Acetoxychavicol acetate inhibits NLRP3-dependent inflammasome activation via mitochondrial ROS suppression.Int Immunol. 2021 Jun 18;33(7):373-386. doi: 10.1093/intimm/dxab016. Int Immunol. 2021. PMID: 33830232
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
A Synthetic Small Molecule F240B Decreases NLRP3 Inflammasome Activation by Autophagy Induction.Front Immunol. 2020 Dec 18;11:607564. doi: 10.3389/fimmu.2020.607564. eCollection 2020. Front Immunol. 2020. PMID: 33424855 Free PMC article.
-
Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors.J Autoimmun. 2020 Sep;113:102515. doi: 10.1016/j.jaut.2020.102515. Epub 2020 Jul 20. J Autoimmun. 2020. PMID: 32703754 Review.
-
Loop Between NLRP3 Inflammasome and Reactive Oxygen Species.Antioxid Redox Signal. 2022 Apr;36(10-12):784-796. doi: 10.1089/ars.2020.8257. Epub 2022 Jan 17. Antioxid Redox Signal. 2022. PMID: 34538111 Review.
Cited by
-
Antiinflammation Derived Suzuki-Coupled Fenbufens as COX-2 Inhibitors: Minilibrary Construction and Bioassay.Molecules. 2022 Apr 29;27(9):2850. doi: 10.3390/molecules27092850. Molecules. 2022. PMID: 35566202 Free PMC article.
-
Chondroitin and glucosamine sulphate reduced proinflammatory molecules in the DRG and improved axonal function of injured sciatic nerve of rats.Sci Rep. 2022 Feb 24;12(1):3196. doi: 10.1038/s41598-022-06554-4. Sci Rep. 2022. PMID: 35210446 Free PMC article.
-
Models of gouty nephropathy: exploring disease mechanisms and identifying potential therapeutic targets.Front Med (Lausanne). 2024 Feb 29;11:1305431. doi: 10.3389/fmed.2024.1305431. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38487029 Free PMC article. Review.
-
Physiological and Pathological Roles of Mammalian NEK7.Front Physiol. 2020 Dec 7;11:606996. doi: 10.3389/fphys.2020.606996. eCollection 2020. Front Physiol. 2020. PMID: 33364979 Free PMC article. Review.
-
Glucosamine Improves Non-Alcoholic Fatty Liver Disease Induced by High-Fat and High-Sugar Diet through Regulating Intestinal Barrier Function, Liver Inflammation, and Lipid Metabolism.Molecules. 2023 Oct 3;28(19):6918. doi: 10.3390/molecules28196918. Molecules. 2023. PMID: 37836761 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

