Repertoires of G protein-coupled receptors for Ciona-specific neuropeptides
- PMID: 30936317
- PMCID: PMC6475428
- DOI: 10.1073/pnas.1816640116
Repertoires of G protein-coupled receptors for Ciona-specific neuropeptides
Abstract
Neuropeptides play pivotal roles in various biological events in the nervous, neuroendocrine, and endocrine systems, and are correlated with both physiological functions and unique behavioral traits of animals. Elucidation of functional interaction between neuropeptides and receptors is a crucial step for the verification of their biological roles and evolutionary processes. However, most receptors for novel peptides remain to be identified. Here, we show the identification of multiple G protein-coupled receptors (GPCRs) for species-specific neuropeptides of the vertebrate sister group, Ciona intestinalis Type A, by combining machine learning and experimental validation. We developed an original peptide descriptor-incorporated support vector machine and used it to predict 22 neuropeptide-GPCR pairs. Of note, signaling assays of the predicted pairs identified 1 homologous and 11 Ciona-specific neuropeptide-GPCR pairs for a 41% hit rate: the respective GPCRs for Ci-GALP, Ci-NTLP-2, Ci-LF-1, Ci-LF-2, Ci-LF-5, Ci-LF-6, Ci-LF-7, Ci-LF-8, Ci-YFV-1, and Ci-YFV-3. Interestingly, molecular phylogenetic tree analysis revealed that these receptors, excluding the Ci-GALP receptor, were evolutionarily unrelated to any other known peptide GPCRs, confirming that these GPCRs constitute unprecedented neuropeptide receptor clusters. Altogether, these results verified the neuropeptide-GPCR pairs in the protochordate and evolutionary lineages of neuropeptide GPCRs, and pave the way for investigating the endogenous roles of novel neuropeptides in the closest relatives of vertebrates and the evolutionary processes of neuropeptidergic systems throughout chordates. In addition, the present study also indicates the versatility of the machine-learning-assisted strategy for the identification of novel peptide-receptor pairs in various organisms.
Keywords: G protein-coupled receptor; deorphanization; machine learning; neuropeptide; peptide descriptor.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




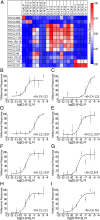

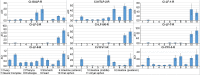

Similar articles
-
Omics Studies for the Identification of Ascidian Peptides, Cognate Receptors, and Their Relevant Roles in Ovarian Follicular Development.Front Endocrinol (Lausanne). 2022 Mar 7;13:858885. doi: 10.3389/fendo.2022.858885. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35321341 Free PMC article. Review.
-
Kobayashi Award 2021: Neuropeptides, receptors, and follicle development in the ascidian, Ciona intestinalis Type A: New clues to the evolution of chordate neuropeptidergic systems from biological niches.Gen Comp Endocrinol. 2023 Jun 1;337:114262. doi: 10.1016/j.ygcen.2023.114262. Epub 2023 Mar 15. Gen Comp Endocrinol. 2023. PMID: 36925021
-
The significance of Ciona intestinalis as a stem organism in integrative studies of functional evolution of the chordate endocrine, neuroendocrine, and nervous systems.Gen Comp Endocrinol. 2016 Feb 1;227:101-8. doi: 10.1016/j.ygcen.2015.05.010. Epub 2015 May 29. Gen Comp Endocrinol. 2016. PMID: 26031189 Review.
-
Impact of Machine Learning-Associated Research Strategies on the Identification of Peptide-Receptor Interactions in the Post-Omics Era.Neuroendocrinology. 2023;113(2):251-261. doi: 10.1159/000518572. Epub 2021 Jul 26. Neuroendocrinology. 2023. PMID: 34348315 Review.
-
Neuropeptides and their receptors of the protochordate, Ciona intestinalis: the evolutionary origin of vertebrate neuropeptides.Acta Biol Hung. 2008;59 Suppl:237-9. doi: 10.1556/ABiol.59.2008.Suppl.33. Acta Biol Hung. 2008. PMID: 18652397
Cited by
-
Lack of membrane sex steroid receptors for mediating rapid endocrine responses in molluscan nervous systems.Front Endocrinol (Lausanne). 2024 Aug 12;15:1458422. doi: 10.3389/fendo.2024.1458422. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39188914 Free PMC article.
-
Omics Studies for the Identification of Ascidian Peptides, Cognate Receptors, and Their Relevant Roles in Ovarian Follicular Development.Front Endocrinol (Lausanne). 2022 Mar 7;13:858885. doi: 10.3389/fendo.2022.858885. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35321341 Free PMC article. Review.
-
Characterization of a putative orexin receptor in Ciona intestinalis sheds light on the evolution of the orexin/hypocretin system in chordates.Sci Rep. 2024 Apr 2;14(1):7690. doi: 10.1038/s41598-024-56508-1. Sci Rep. 2024. PMID: 38565870 Free PMC article.
-
Editorial: Endocrine and Neuroendocrine Systems of Invertebrate Deuterostomes.Front Endocrinol (Lausanne). 2019 Nov 1;10:755. doi: 10.3389/fendo.2019.00755. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31736882 Free PMC article. No abstract available.
-
Draft genome of Tanacetum cinerariifolium, the natural source of mosquito coil.Sci Rep. 2019 Dec 3;9(1):18249. doi: 10.1038/s41598-019-54815-6. Sci Rep. 2019. PMID: 31796833 Free PMC article.
References
-
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

