Induction of a Spindle-Assembly-Competent M Phase in Xenopus Egg Extracts
- PMID: 30930041
- PMCID: PMC6532407
- DOI: 10.1016/j.cub.2019.02.061
Induction of a Spindle-Assembly-Competent M Phase in Xenopus Egg Extracts
Abstract
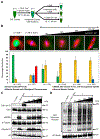
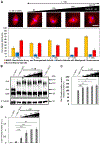
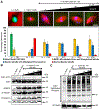
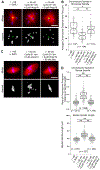
Normal mitotic spindle assembly is a prerequisite for faithful chromosome segregation and unperturbed cell-cycle progression. Precise functioning of the spindle machinery relies on conserved architectural features, such as focused poles, chromosome alignment at the metaphase plate, and proper spindle length. These morphological requirements can be achieved only within a compositionally distinct cytoplasm that results from cell-cycle-dependent regulation of specific protein levels and specific post-translational modifications. Here, we used cell-free extracts derived from Xenopus laevis eggs to recapitulate different phases of the cell cycle in vitro and to determine which components are required to render interphase cytoplasm spindle-assembly competent in the absence of protein translation. We found that addition of a nondegradable form of the master cell-cycle regulator cyclin B1 can indeed induce some biochemical and phenomenological characteristics of mitosis, but cyclin B1 alone is insufficient and actually deleterious at high levels for normal spindle assembly. In contrast, addition of a phosphomimetic form of the Greatwall-kinase effector Arpp19 with a specific concentration of nondegradable cyclin B1 rescued spindle bipolarity but resulted in larger-than-normal bipolar spindles with a misalignment of chromosomes. Both were corrected by the addition of exogenous Xkid (Xenopus homolog of human Kid/KIF22), indicating a role for this chromokinesin in regulating spindle length. These observations suggest that, of the many components degraded at mitotic exit and then replenished during the subsequent interphase, only a few are required to induce a cell-cycle transition that produces a spindle-assembly-competent cytoplasm.
Keywords: Greatwall kinase; M phase; Xenopus egg extract; cell cycle; chromokinesin; cyclin; mitosis; spindle assembly.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Investigating mitotic spindle assembly and function in vitro using Xenopus laevis egg extracts.Nat Protoc. 2006;1(5):2305-14. doi: 10.1038/nprot.2006.396. Nat Protoc. 2006. PMID: 17406472
-
Potential roles of the nucleotide exchange factor ECT2 and Cdc42 GTPase in spindle assembly in Xenopus egg cell-free extracts.J Cell Biochem. 2003 Dec 1;90(5):892-900. doi: 10.1002/jcb.10750. J Cell Biochem. 2003. PMID: 14624449
-
MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts.J Cell Biol. 1997 Mar 10;136(5):1091-7. doi: 10.1083/jcb.136.5.1091. J Cell Biol. 1997. PMID: 9060473 Free PMC article.
-
Methods for studying spindle assembly and chromosome condensation in Xenopus egg extracts.Methods Mol Biol. 2006;322:459-74. doi: 10.1007/978-1-59745-000-3_33. Methods Mol Biol. 2006. PMID: 16739744 Review.
-
Centrosomes and the art of mitotic spindle maintenance.Int Rev Cell Mol Biol. 2014;313:179-217. doi: 10.1016/B978-0-12-800177-6.00006-2. Int Rev Cell Mol Biol. 2014. PMID: 25376493 Review.
Cited by
-
Quantitative mapping of transcriptome and proteome dynamics during polarization of human iPSC-derived neurons.Elife. 2020 Sep 17;9:e58124. doi: 10.7554/eLife.58124. Elife. 2020. PMID: 32940601 Free PMC article.
References
-
- Peters JM (2006). The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7, 644–656. - PubMed
-
- Murray AW, Solomon MJ, and Kirschner MW (1989). The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339, 280–286. - PubMed
-
- Nurse P (1990). Universal control mechanism regulating onset of M-phase. Nature 344, 503–508. - PubMed
-
- Pines J, and Hunter T (1990). Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 346, 760–763. - PubMed
-
- Boekhout M, and Wolthuis R (2015). Nek2A destruction marks APC/C activation at the prophase-to-prometaphase transition by spindle-checkpoint-restricted Cdc20. J Cell Sci 128, 1639–1653. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

