Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis
- PMID: 30912096
- PMCID: PMC6530577
- DOI: 10.1055/s-0039-1681032
Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis
Abstract
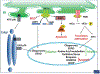
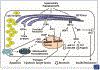
Endoplasmic reticulum (ER) stress is a major contributor to liver disease and hepatic fibrosis, but the role it plays varies depending on the cause and progression of the disease. Furthermore, ER stress plays a distinct role in hepatocytes versus hepatic stellate cells (HSCs), which adds to the complexity of understanding ER stress and its downstream signaling through the unfolded protein response (UPR) in liver disease. Here, the authors focus on the current literature of ER stress in nonalcoholic and alcoholic fatty liver diseases, how ER stress impacts hepatocyte injury, and the role of ER stress in HSC activation and hepatic fibrosis. This review provides insight into the complex signaling and regulation of the UPR, parallels and distinctions between different liver diseases, and how ER stress may be targeted as an antisteatotic or antifibrotic therapy to limit the progression of liver disease.
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Conflict of interest statement
None.
Figures

Similar articles
-
Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2.Gastroenterology. 2016 Jan;150(1):181-193.e8. doi: 10.1053/j.gastro.2015.09.039. Epub 2015 Oct 3. Gastroenterology. 2016. PMID: 26435271
-
Adenoviral CCN gene transfers induce in vitro and in vivo endoplasmic reticulum stress and unfolded protein response.Biochim Biophys Acta. 2016 Nov;1863(11):2604-2612. doi: 10.1016/j.bbamcr.2016.07.006. Epub 2016 Jul 22. Biochim Biophys Acta. 2016. PMID: 27452908
-
Endoplasmic reticulum proteostasis in hepatic steatosis.Nat Rev Endocrinol. 2016 Dec;12(12):710-722. doi: 10.1038/nrendo.2016.124. Epub 2016 Aug 12. Nat Rev Endocrinol. 2016. PMID: 27516341 Review.
-
High-mobility group box 1 induces endoplasmic reticulum stress and activates hepatic stellate cells.Lab Invest. 2018 Sep;98(9):1200-1210. doi: 10.1038/s41374-018-0085-9. Epub 2018 Jun 29. Lab Invest. 2018. PMID: 29959419
-
Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis.Inflamm Res. 2015 Jan;64(1):1-7. doi: 10.1007/s00011-014-0772-y. Epub 2014 Oct 7. Inflamm Res. 2015. PMID: 25286903 Review.
Cited by
-
XBP1-mediated activation of the STING signalling pathway in macrophages contributes to liver fibrosis progression.JHEP Rep. 2022 Aug 18;4(11):100555. doi: 10.1016/j.jhepr.2022.100555. eCollection 2022 Nov. JHEP Rep. 2022. PMID: 36185574 Free PMC article.
-
Loss of heat shock factor 1 promotes hepatic stellate cell activation and drives liver fibrosis.Hepatol Commun. 2022 Oct;6(10):2781-2797. doi: 10.1002/hep4.2058. Epub 2022 Aug 9. Hepatol Commun. 2022. PMID: 35945902 Free PMC article.
-
Characterizing the impact of simvastatin co-treatment of cell specific TCDD-induced gene expression and systemic toxicity.Sci Rep. 2023 Oct 3;13(1):16598. doi: 10.1038/s41598-023-42972-8. Sci Rep. 2023. PMID: 37789023 Free PMC article.
-
Nonalcoholic Steatohepatitis Promoting Kinases.Semin Liver Dis. 2020 Nov;40(4):346-357. doi: 10.1055/s-0040-1713115. Epub 2020 Jun 11. Semin Liver Dis. 2020. PMID: 32526787 Free PMC article. Review.
-
Exploring the Role of Endoplasmic Reticulum Stress in Hepatocellular Carcinoma through mining of the Human Protein Atlas.Biology (Basel). 2021 Jul 9;10(7):640. doi: 10.3390/biology10070640. Biology (Basel). 2021. PMID: 34356495 Free PMC article.
References
-
- Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018; 4(01):16. - PubMed
-
- Yoshiuchi K, Kaneto H, Matsuoka TA, et al. Pioglitazone reduces ER stress in the liver: direct monitoring of in vivo ER stress using ER stress-activated indicator transgenic mice. Endocr J 2009; 56 (09):1103–1111 - PubMed
-
- Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006; 147(02):943–951 - PubMed

