Bridging human chaperonopathies and microbial chaperonins
- PMID: 30911678
- PMCID: PMC6420498
- DOI: 10.1038/s42003-019-0318-5
Bridging human chaperonopathies and microbial chaperonins
Abstract
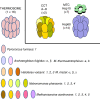
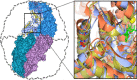
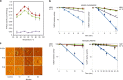
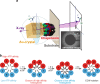
Chaperonins are molecular chaperones that play critical physiological roles, but they can be pathogenic. Malfunctional chaperonins cause chaperonopathies of great interest within various medical specialties. Although the clinical-genetic aspects of many chaperonopathies are known, the molecular mechanisms causing chaperonin failure and tissue lesions are poorly understood. Progress is necessary to improve treatment, and experimental models that mimic the human situation provide a promising solution. We present two models: one prokaryotic (the archaeon Pyrococcus furiosus) with eukaryotic-like chaperonins and one eukaryotic (Chaetomium thermophilum), both convenient for isolation-study of chaperonins, and report illustrative results pertaining to a pathogenic mutation of CCT5.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Chaperones and protein folding in the archaea.Biochem Soc Trans. 2009 Feb;37(Pt 1):46-51. doi: 10.1042/BST0370046. Biochem Soc Trans. 2009. PMID: 19143600 Review.
-
Kinetics and binding sites for interaction of the prefoldin with a group II chaperonin: contiguous non-native substrate and chaperonin binding sites in the archaeal prefoldin.J Biol Chem. 2004 Jul 23;279(30):31788-95. doi: 10.1074/jbc.M402889200. Epub 2004 May 15. J Biol Chem. 2004. PMID: 15145959
-
Prokaryotic Chaperonins as Experimental Models for Elucidating Structure-Function Abnormalities of Human Pathogenic Mutant Counterparts.Front Mol Biosci. 2017 Jan 9;3:84. doi: 10.3389/fmolb.2016.00084. eCollection 2016. Front Mol Biosci. 2017. PMID: 28119916 Free PMC article. Review.
-
Overexpression of prefoldin from the hyperthermophilic archaeum Pyrococcus horikoshii OT3 endowed Escherichia coli with organic solvent tolerance.Appl Microbiol Biotechnol. 2008 Jun;79(3):443-9. doi: 10.1007/s00253-008-1450-1. Epub 2008 Apr 29. Appl Microbiol Biotechnol. 2008. PMID: 18443786
-
A Multipronged Method for Unveiling Subtle Structural-Functional Defects of Mutant Chaperone Molecules Causing Human Chaperonopathies.Methods Mol Biol. 2019;1873:69-92. doi: 10.1007/978-1-4939-8820-4_5. Methods Mol Biol. 2019. PMID: 30341604
Cited by
-
Minimal Yet Powerful: The Role of Archaeal Small Heat Shock Proteins in Maintaining Protein Homeostasis.Front Mol Biosci. 2022 May 12;9:832160. doi: 10.3389/fmolb.2022.832160. eCollection 2022. Front Mol Biosci. 2022. PMID: 35647036 Free PMC article. Review.
References
-
- Macario, A. J. L., Conway de Macario, E. & Cappello, F. The Chaperonopathies. Diseases with Defective Molecular Chaperones. (Springer, Dordrecht, Heidelberg, New York, London, 2013). http://link.springer.com/book/10.1007%2F978-94-007-4667-1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

