Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function
- PMID: 30910955
- PMCID: PMC6462103
- DOI: 10.1073/pnas.1901376116
Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function
Abstract
Cellular metabolism and signaling pathways are key regulators to determine conventional T cell fate and function, but little is understood about the role of cell metabolism for natural killer T (NKT) cell survival, proliferation, and function. We found that NKT cells operate distinct metabolic programming from CD4 T cells. NKT cells are less efficient in glucose uptake than CD4 T cells with or without activation. Gene-expression data revealed that, in NKT cells, glucose is preferentially metabolized by the pentose phosphate pathway and mitochondria, as opposed to being converted into lactate. In fact, glucose is essential for the effector functions of NKT cells and a high lactate environment is detrimental for NKT cell survival and proliferation. Increased glucose uptake and IFN-γ expression in NKT cells is inversely correlated with bacterial loads in response to bacterial infection, further supporting the significance of glucose metabolism for NKT cell function. We also found that promyelocytic leukemia zinc finger seemed to play a role in regulating NKT cells' glucose metabolism. Overall, our study reveals that NKT cells use distinct arms of glucose metabolism for their survival and function.
Keywords: NKT; OXPHOS; PLZF; glucose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
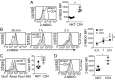
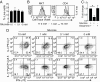

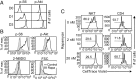

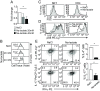

Similar articles
-
Reactive Oxygen Species Regulate the Inflammatory Function of NKT Cells through Promyelocytic Leukemia Zinc Finger.J Immunol. 2017 Nov 15;199(10):3478-3487. doi: 10.4049/jimmunol.1700567. Epub 2017 Oct 11. J Immunol. 2017. PMID: 29021374 Free PMC article.
-
Id3 Restricts γδ NKT Cell Expansion by Controlling Egr2 and c-Myc Activity.J Immunol. 2018 Sep 1;201(5):1452-1459. doi: 10.4049/jimmunol.1800106. Epub 2018 Jul 16. J Immunol. 2018. PMID: 30012846 Free PMC article.
-
PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals.J Immunol. 2010 Jun 15;184(12):6746-55. doi: 10.4049/jimmunol.1000776. Epub 2010 May 21. J Immunol. 2010. PMID: 20495068
-
Pak2 Controls Acquisition of NKT Cell Fate by Regulating Expression of the Transcription Factors PLZF and Egr2.J Immunol. 2015 Dec 1;195(11):5272-84. doi: 10.4049/jimmunol.1501367. Epub 2015 Oct 30. J Immunol. 2015. PMID: 26519537 Free PMC article.
-
Natural killer T cells within the liver: conductors of the hepatic immune orchestra.Dig Dis. 2010;28(1):7-13. doi: 10.1159/000282059. Epub 2010 May 7. Dig Dis. 2010. PMID: 20460885 Review.
Cited by
-
Targeting L-Lactate Metabolism to Overcome Resistance to Immune Therapy of Melanoma and Other Tumor Entities.J Oncol. 2019 Nov 3;2019:2084195. doi: 10.1155/2019/2084195. eCollection 2019. J Oncol. 2019. PMID: 31781212 Free PMC article. Review.
-
Research progress of abnormal lactate metabolism and lactate modification in immunotherapy of hepatocellular carcinoma.Front Oncol. 2023 Jan 6;12:1063423. doi: 10.3389/fonc.2022.1063423. eCollection 2022. Front Oncol. 2023. PMID: 36686771 Free PMC article. Review.
-
Lactate Metabolism and Immune Modulation in Breast Cancer: A Focused Review on Triple Negative Breast Tumors.Front Oncol. 2020 Nov 26;10:598626. doi: 10.3389/fonc.2020.598626. eCollection 2020. Front Oncol. 2020. PMID: 33324565 Free PMC article. Review.
-
Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells.Nat Nanotechnol. 2022 Jan;17(1):98-106. doi: 10.1038/s41565-021-01000-4. Epub 2021 Nov 18. Nat Nanotechnol. 2022. PMID: 34795441 Free PMC article.
-
OX40+ plasmacytoid dendritic cells in the tumor microenvironment promote antitumor immunity.J Clin Invest. 2020 Jul 1;130(7):3528-3542. doi: 10.1172/JCI131992. J Clin Invest. 2020. PMID: 32182225 Free PMC article.
References
-
- Arase H, Arase N, Nakagawa K, Good RA, Onoé K. NK1.1+ CD4+ CD8- thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–310. - PubMed
-
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. - PubMed
-
- Zeng Z, et al. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. - PubMed
-
- Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. - PubMed
-
- Zhang L, et al. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol. 2014;193:1759–1765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

