Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy
- PMID: 30910484
- PMCID: PMC6492224
- DOI: 10.1016/j.ebiom.2019.03.033
Tau antibody chimerization alters its charge and binding, thereby reducing its cellular uptake and efficacy
Abstract
Background: Bringing antibodies from pre-clinical studies to human trials requires humanization, but this process may alter properties that are crucial for efficacy. Since pathological tau protein is primarily intraneuronal in Alzheimer's disease, the most efficacious antibodies should work both intra- and extracellularly. Thus, changes which impact uptake or antibody binding will affect antibody efficacy.
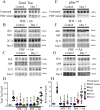
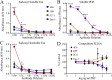
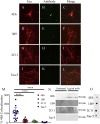
Methods: Initially, we examined four tau mouse monoclonal antibodies with naturally differing charges. We quantified their neuronal uptake, and efficacy in preventing toxicity and pathological seeding induced by human-derived pathological tau. Later, we generated a human chimeric 4E6 (h4E6), an antibody with well documented efficacy in multiple tauopathy models. We compared the uptake and efficacy of unmodified and chimeric antibodies in neuronal and differentiated neuroblastoma cultures. Further, we analyzed tau binding using ELISA assays.
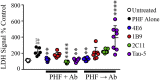
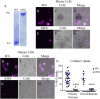
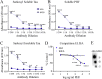
Findings: Neuronal uptake of tau antibodies and their efficacy strongly depends on antibody charge. Additionally, their ability to prevent tau toxicity and seeding of tau pathology does not necessarily go together. Particularly, chimerization of 4E6 increased its charge from 6.5 to 9.6, which blocked its uptake into human and mouse cells. Furthermore, h4E6 had altered binding characteristics despite intact binding sites, compared to the mouse antibody. Importantly, these changes in uptake and binding substantially decreased its efficacy in preventing tau toxicity, although under certain conditions it did prevent pathological seeding of tau.
Conclusions: These results indicate that efficacy of chimeric/humanized tau antibodies should be thoroughly characterized prior to clinical trials, which may require further engineering to maintain or improve their therapeutic potential. FUND: National Institutes of Health (NS077239, AG032611, R24OD18340, R24OD018339 and RR027990, Alzheimer's Association (2016-NIRG-397228) and Blas Frangione Foundation.
Keywords: Alzheimer's disease; Antibody engineering; Immunotherapy; Neuroblastoma; Neuron; Tau protein; Vaccine development.
Copyright © 2019. Published by Elsevier B.V.
Figures




Similar articles
-
Affinity of Tau antibodies for solubilized pathological Tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy.Mol Neurodegener. 2016 Aug 30;11(1):62. doi: 10.1186/s13024-016-0126-z. Mol Neurodegener. 2016. PMID: 27578006 Free PMC article.
-
Humanized monoclonal antibody armanezumab specific to N-terminus of pathological tau: characterization and therapeutic potency.Mol Neurodegener. 2017 May 5;12(1):33. doi: 10.1186/s13024-017-0172-1. Mol Neurodegener. 2017. PMID: 28472993 Free PMC article.
-
Novel Phospho-Tau Monoclonal Antibody Generated Using a Liposomal Vaccine, with Enhanced Recognition of a Conformational Tauopathy Epitope.J Alzheimers Dis. 2017;56(2):585-599. doi: 10.3233/JAD-160695. J Alzheimers Dis. 2017. PMID: 28035925 Free PMC article.
-
Tau neurotoxicity and rescue in animal models of human Tauopathies.Curr Opin Neurobiol. 2016 Feb;36:52-8. doi: 10.1016/j.conb.2015.09.004. Epub 2015 Sep 29. Curr Opin Neurobiol. 2016. PMID: 26431808 Review.
-
Tau Immunotherapy.Neurodegener Dis. 2016;16(1-2):34-8. doi: 10.1159/000440842. Epub 2015 Nov 10. Neurodegener Dis. 2016. PMID: 26551002 Free PMC article. Review.
Cited by
-
Tau immunotherapies: Lessons learned, current status and future considerations.Neuropharmacology. 2020 Sep 15;175:108104. doi: 10.1016/j.neuropharm.2020.108104. Epub 2020 Apr 28. Neuropharmacology. 2020. PMID: 32360477 Free PMC article. Review.
-
Single-Domain Antibody-Based Protein Degrader for Synucleinopathies.bioRxiv [Preprint]. 2024 Apr 30:2024.03.11.584473. doi: 10.1101/2024.03.11.584473. bioRxiv. 2024. Update in: Mol Neurodegener. 2024 May 31;19(1):44. doi: 10.1186/s13024-024-00730-y. PMID: 38558982 Free PMC article. Updated. Preprint.
-
Conformation-selective tau monoclonal antibodies inhibit tau pathology in primary neurons and a mouse model of Alzheimer's disease.Mol Neurodegener. 2020 Nov 4;15(1):64. doi: 10.1186/s13024-020-00404-5. Mol Neurodegener. 2020. PMID: 33148293 Free PMC article.
-
Current Status of Clinical Trials on Tau Immunotherapies.Drugs. 2021 Jul;81(10):1135-1152. doi: 10.1007/s40265-021-01546-6. Epub 2021 Jun 8. Drugs. 2021. PMID: 34101156 Free PMC article. Review.
-
Dynamics of Internalization and Intracellular Interaction of Tau Antibodies and Human Pathological Tau Protein in a Human Neuron-Like Model.Front Neurol. 2020 Nov 26;11:602292. doi: 10.3389/fneur.2020.602292. eCollection 2020. Front Neurol. 2020. PMID: 33324339 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

