2,4 Dinitrophenol as Medicine
- PMID: 30909602
- PMCID: PMC6468406
- DOI: 10.3390/cells8030280
2,4 Dinitrophenol as Medicine
Abstract
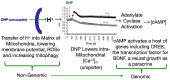
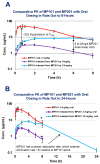
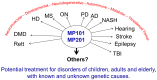
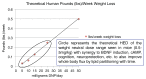
In the sanctity of pure drug discovery, objective reasoning can become clouded when pursuing ideas that appear unorthodox, but are spot on physiologically. To put this into historical perspective, it was an unorthodox idea in the 1950's to suggest that warfarin, a rat poison, could be repositioned into a breakthrough drug in humans to protect against strokes as a blood thinner. Yet it was approved in 1954 as Coumadin® and has been prescribed to billions of patients as a standard of care. Similarly, no one can forget the horrific effects of thalidomide, prescribed or available without a prescription, as both a sleeping pill and "morning sickness" anti-nausea medication targeting pregnant women in the 1950's. The "thalidomide babies" became the case-in-point for the need of strict guidelines by the U.S. Food & Drug Administration (FDA) or full multi-species teratogenicity testing before drug approval. More recently it was found that thalidomide is useful in graft versus host disease, leprosy and resistant tuberculosis treatment, and as an anti-angiogenesis agent as a breakthrough drug for multiple myeloma (except for pregnant female patients). Decades of diabetes drug discovery research has historically focused on every possible angle, except, the energy-out side of the equation, namely, raising mitochondrial energy expenditure with chemical uncouplers. The idea of "social responsibility" allowed energy-in agents to be explored and the portfolio is robust with medicines of insulin sensitizers, insulin analogues, secretagogues, SGLT2 inhibitors, etc., but not energy-out medicines. The primary reason? It appeared unorthodox, to return to exploring a drug platform used in the 1930s in over 100,000 obese patients used for weight loss. This is over 80-years ago and prior to Dr Peter Mitchell explaining the mechanism of how mitochondrial uncouplers, like 2,4-dinitrophenol (DNP) even worked by three decades later in 1961. Although there is a clear application for metabolic disease, it was not until recently that this platform was explored for its merit at very low, weight-neutral doses, for treating insidious human illnesses and completely unrelated to weight reduction. It is known that mitochondrial uncouplers specifically target the entire organelle's physiology non-genomically. It has been known for years that many neuromuscular and neurodegenerative diseases are associated with overt production of reactive oxygen species (ROSs), a rise in isoprostanes (biomarker of mitochondrial ROSs in urine or blood) and poor calcium (Ca2+) handing. It has also been known that mitochondrial uncouplers lower ROS production and Ca2+ overload. There is evidence that elevation of isoprostanes precedes disease onset, in Alzheimer's Disease (AD). It is also curious, why so many neurodegenerative diseases of known and unknown etiology start at mid-life or later, such as Multiple Sclerosis (MS), Huntington Disease (HD), AD, Parkinson Disease, and Amyotrophic Lateral Sclerosis (ALS). Is there a relationship to a buildup of mutations that are sequestered over time due to ROSs exceeding the rate of repair? If ROS production were managed, could disease onset due to aging be delayed or prevented? Is it possible that most, if not all neurodegenerative diseases are manifested through mitochondrial dysfunction? Although DNP, a historic mitochondrial uncoupler, was used in the 1930s at high doses for obesity in well over 100,000 humans, and so far, it has never been an FDA-approved drug. This review will focus on the application of using DNP, but now, repositioned as a potential disease-modifying drug for a legion of insidious diseases at much lower and paradoxically, weight neutral doses. DNP will be addressed as a treatment for "metabesity", an emerging term related to the global comorbidities associated with the over-nutritional phenotype; obesity, diabetes, nonalcoholic steatohepatitis (NASH), metabolic syndrome, cardiovascular disease, but including neurodegenerative disorders and accelerated aging. Some unexpected drug findings will be discussed, such as DNP's induction of neurotrophic growth factors involved in neuronal heath, learning and cognition. For the first time in 80's years, the FDA has granted (to Mitochon Pharmaceutical, Inc., Blue Bell, PA, USA) an open Investigational New Drug (IND) approval to begin rigorous clinical testing of DNP for safety and tolerability, including for the first ever, pharmacokinetic profiling in humans. Successful completion of Phase I clinical trial will open the door to explore the merits of DNP as a possible treatment of people with many truly unmet medical needs, including those suffering from HD, MS, PD, AD, ALS, Duchenne Muscular Dystrophy (DMD), and Traumatic Brain Injury (TBI).
Keywords: 2,4-dinitrophenol (DNP); Brain-derived neurotrophic factor (BDNF); Duchenne Muscular Dystrophy; Huntington’s Disease; Multiple Sclerosis; Traumatic Brain Injury; anti-aging; metabesity; mitochondrial uncoupler; neurodegeneration.
Conflict of interest statement
John G. Geisler is a founder and shareholder of Mitochon Pharmaceuticals, Inc. MP201 (DNP prodrug) has pending U.S. patent applications (15/451,938 and 62/693,142), as well as DNP/MP101 (15/002,531 and 15/357,412). The FDA has awarded Mitochon Pharmaceuticals Orphan Designation for DNP/MP101 for Huntington’s Disease.
Figures
Similar articles
-
Disease modifying mitochondrial uncouplers, MP101, and a slow release ProDrug, MP201, in models of Multiple Sclerosis.Neurochem Int. 2019 Dec;131:104561. doi: 10.1016/j.neuint.2019.104561. Epub 2019 Oct 1. Neurochem Int. 2019. PMID: 31585135
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
-
The mitochondrial uncoupler DNP triggers brain cell mTOR signaling network reprogramming and CREB pathway up-regulation.J Neurochem. 2015 Aug;134(4):677-92. doi: 10.1111/jnc.13176. Epub 2015 Jun 19. J Neurochem. 2015. PMID: 26010875 Free PMC article.
-
DNP, mitochondrial uncoupling, and neuroprotection: A little dab'll do ya.Alzheimers Dement. 2017 May;13(5):582-591. doi: 10.1016/j.jalz.2016.08.001. Epub 2016 Sep 4. Alzheimers Dement. 2017. PMID: 27599210 Free PMC article.
-
Mecasermin rinfabate: insulin-like growth factor-I/insulin-like growth factor binding protein-3, mecaserimin rinfibate, rhIGF-I/rhIGFBP-3.Drugs R D. 2005;6(2):120-7. doi: 10.2165/00126839-200506020-00008. Drugs R D. 2005. PMID: 15777106 Review.
Cited by
-
Anthelmintics nitazoxanide protects against experimental hyperlipidemia and hepatic steatosis in hamsters and mice.Acta Pharm Sin B. 2022 Mar;12(3):1322-1338. doi: 10.1016/j.apsb.2021.09.009. Epub 2021 Sep 17. Acta Pharm Sin B. 2022. PMID: 35530137 Free PMC article.
-
Pioglitazone Is a Mild Carrier-Dependent Uncoupler of Oxidative Phosphorylation and a Modulator of Mitochondrial Permeability Transition.Pharmaceuticals (Basel). 2021 Oct 14;14(10):1045. doi: 10.3390/ph14101045. Pharmaceuticals (Basel). 2021. PMID: 34681269 Free PMC article.
-
The Link between Chronic Stress and Accelerated Aging.Biomedicines. 2020 Jul 7;8(7):198. doi: 10.3390/biomedicines8070198. Biomedicines. 2020. PMID: 32645916 Free PMC article. Review.
-
Noncoupled Mitochondrial Respiration as Therapeutic Approach for the Treatment of Metabolic Diseases: Focus on Transgenic Animal Models.Int J Mol Sci. 2023 Nov 18;24(22):16491. doi: 10.3390/ijms242216491. Int J Mol Sci. 2023. PMID: 38003681 Free PMC article. Review.
-
Mitochondrial pathways in human health and aging.Mitochondrion. 2020 Sep;54:72-84. doi: 10.1016/j.mito.2020.07.007. Epub 2020 Jul 30. Mitochondrion. 2020. PMID: 32738358 Free PMC article. Review.
References
-
- Wosniak J., Jr., Santos C.X., Kowaltowski A.J., Laurindo F.R. Cross-talk between mitochondria and NADPH oxidase: Effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid. Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. - DOI - PubMed
-
- Armand R., Channon J.Y., Kintner J., White K.A., Miselis K.A., Perez R.P., Lewis L.D. The effects of ethidium bromide induced loss of mitochondrial DNA on mitochondrial phenotype and ultrastructure in a human leukemia T-cell line (MOLT-4 cells) Toxicol. Appl. Pharmacol. 2004;196:68–79. doi: 10.1016/j.taap.2003.12.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

