Recombinant RGD-disintegrin DisBa-01 blocks integrin αvβ3 and impairs VEGF signaling in endothelial cells
- PMID: 30894182
- PMCID: PMC6425665
- DOI: 10.1186/s12964-019-0339-1
Recombinant RGD-disintegrin DisBa-01 blocks integrin αvβ3 and impairs VEGF signaling in endothelial cells
Abstract
Background: Integrins mediate cell adhesion, migration, and survival by connecting the intracellular machinery with the surrounding extracellular matrix. Previous studies demonstrated the interaction between αvβ3 integrin and VEGF type 2 receptor (VEGFR2) in VEGF-induced angiogenesis. DisBa-01, a recombinant His-tag fusion, RGD-disintegrin from Bothrops alternatus snake venom, binds to αvβ3 integrin with nanomolar affinity blocking cell adhesion to the extracellular matrix. Here we present in vitro evidence of a direct interference of DisBa-01 with αvβ3/VEGFR2 cross-talk and its downstream pathways.
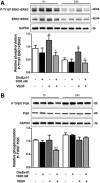
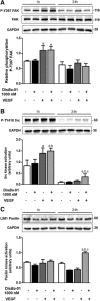
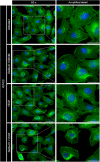
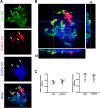
Methods: Human umbilical vein (HUVECs) were cultured in plates coated with fibronectin (FN) or vitronectin (VN) and tested for migration, invasion and proliferation assays in the presence of VEGF, DisBa-01 (1000 nM) or VEGF and DisBa-01 simultaneously. Phosphorylation of αvβ3/VEGFR2 receptors and the activation of intracellular signaling pathways were analyzed by western blotting. Morphological alterations were observed and quantified by fluorescence confocal microscopy.
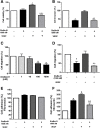
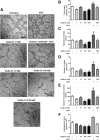
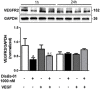
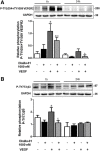
Results: DisBa-01 treatment of endothelial cells inhibited critical steps of VEGF-mediated angiogenesis such as migration, invasion and tubulogenesis. The blockage of αvβ3/VEGFR2 cross-talk by this disintegrin decreases protein expression and phosphorylation of VEGFR2 and β3 integrin subunit, regulates FAK/SrC/Paxillin downstream signals, and inhibits ERK1/2 and PI3K pathways. These events result in actin re-organization and inhibition of HUVEC migration and adhesion. Labelled-DisBa-01 colocalizes with αvβ3 integrin and VEGFR2 in treated cells.
Conclusions: Disintegrin inhibition of αvβ3 integrin blocks VEGFR2 signalling, even in the presence of VEGF, which impairs the angiogenic mechanism. These results improve our understanding concerning the mechanisms of pharmacological inhibition of angiogenesis.
Keywords: Angiogenesis; Cross-talk; DisBa-01; Disintegrin; Extracellular matrix; VEGFR2; αvβ3 integrin.
Conflict of interest statement
Author’s information
All authors are from the Laboratory of Biochemistry and Molecular Biology, Department of Physiological Sciences, Federal University of São Carlos at São Carlos, São Paulo State, Brazil.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read this manuscript and approved for the submission.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Blocking αvβ3 integrin by a recombinant RGD disintegrin impairs VEGF signaling in endothelial cells.Biochimie. 2012 Aug;94(8):1812-20. doi: 10.1016/j.biochi.2012.04.020. Epub 2012 Apr 27. Biochimie. 2012. PMID: 22561350
-
Alternagin-C (ALT-C), a disintegrin-like protein, attenuates alpha2beta1 integrin and VEGF receptor 2 signaling resulting in angiogenesis inhibition.Biochimie. 2020 Jul;174:144-158. doi: 10.1016/j.biochi.2020.04.023. Epub 2020 Apr 30. Biochimie. 2020. PMID: 32360415
-
The Effects of αvβ3 Integrin Blockage in Breast Tumor and Endothelial Cells under Hypoxia In Vitro.Int J Mol Sci. 2022 Feb 3;23(3):1745. doi: 10.3390/ijms23031745. Int J Mol Sci. 2022. PMID: 35163668 Free PMC article.
-
Alternagin-C, a disintegrin-like protein from the venom of Bothrops alternatus, modulates alpha2beta1 integrin-mediated cell adhesion, migration and proliferation.Braz J Med Biol Res. 2005 Oct;38(10):1505-11. doi: 10.1590/s0100-879x2005001000007. Epub 2005 Sep 6. Braz J Med Biol Res. 2005. PMID: 16172743 Review.
-
Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis.Angiogenesis. 2009;12(2):177-85. doi: 10.1007/s10456-009-9141-9. Epub 2009 Mar 8. Angiogenesis. 2009. PMID: 19267251 Free PMC article. Review.
Cited by
-
Integrin-specific hydrogels for growth factor-free vasculogenesis.NPJ Regen Med. 2022 Sep 27;7(1):57. doi: 10.1038/s41536-022-00253-4. NPJ Regen Med. 2022. PMID: 36167724 Free PMC article.
-
Proliferative and Osteogenic Supportive Effect of VEGF-Loaded Collagen-Chitosan Hydrogel System in Bone Marrow Derived Mesenchymal Stem Cells.Pharmaceutics. 2023 Apr 20;15(4):1297. doi: 10.3390/pharmaceutics15041297. Pharmaceutics. 2023. PMID: 37111780 Free PMC article.
-
The complex relationship between integrins and oncolytic herpes Simplex Virus 1 in high-grade glioma therapeutics.Mol Ther Oncolytics. 2022 Jun 6;26:63-75. doi: 10.1016/j.omto.2022.05.013. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 35795093 Free PMC article. Review.
-
Pictolysin-III, a Hemorrhagic Type-III Metalloproteinase Isolated from Bothrops pictus (Serpentes: Viperidae) Venom, Reduces Mitochondrial Respiration and Induces Cytokine Secretion in Epithelial and Stromal Cell Lines.Pharmaceutics. 2023 May 18;15(5):1533. doi: 10.3390/pharmaceutics15051533. Pharmaceutics. 2023. PMID: 37242775 Free PMC article.
-
Biomaterials in Valvular Heart Diseases.Front Bioeng Biotechnol. 2020 Dec 9;8:529244. doi: 10.3389/fbioe.2020.529244. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33425862 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

