Comparative Cardiac Safety of Selective Serotonin Reuptake Inhibitors among Individuals Receiving Maintenance Hemodialysis
- PMID: 30885935
- PMCID: PMC6442344
- DOI: 10.1681/ASN.2018101032
Comparative Cardiac Safety of Selective Serotonin Reuptake Inhibitors among Individuals Receiving Maintenance Hemodialysis
Abstract
Background: Individuals receiving maintenance hemodialysis may be particularly susceptible to the lethal cardiac consequences of drug-induced QT prolongation because they have a substantial cardiovascular disease burden and high level of polypharmacy, as well as recurrent exposure to electrolyte shifts during dialysis. Electrophysiologic data indicate that among the selective serotonin reuptake inhibitors (SSRIs), citalopram and escitalopram prolong the QT interval to the greatest extent. However, the relative cardiac safety of SSRIs in the hemodialysis population is unknown.
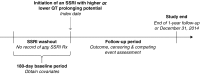
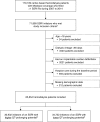
Methods: In this retrospective cohort study, we used data from a cohort of Medicare beneficiaries receiving hemodialysis included in the US Renal Data System registry (2007-2014). We used a new-user design to compare the 1-year risk of sudden cardiac death among hemodialysis patients initiating SSRIs with a higher potential for prolonging the QT interval (citalopram, escitalopram) versus the risk among those initiating SSRIs with lower QT-prolonging potential (fluoxetine, fluvoxamine, paroxetine, sertraline). We estimated adjusted hazard ratios using inverse probability of treatment weighted survival models. Nonsudden cardiac death was treated as a competing event.
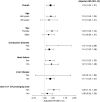
Results: The study included 30,932 (47.1%) hemodialysis patients who initiated SSRIs with higher QT-prolonging potential and 34,722 (52.9%) who initiated SSRIs with lower QT-prolonging potential. Initiation of an SSRI with higher versus lower QT-prolonging potential was associated with higher risk of sudden cardiac death (adjusted hazard ratio, 1.18; 95% confidence interval, 1.05 to 1.31). This association was more pronounced among elderly individuals, females, patients with conduction disorders, and those treated with other non-SSRI QT-prolonging medications.
Conclusions: The heterogeneous QT-prolonging potential of SSRIs may differentially affect cardiac outcomes in the hemodialysis population.
Keywords: SSRIs; Safety; Sudden cardiac death; hemodialysis.
Copyright © 2019 by the American Society of Nephrology.
Figures


Comment in
-
Piecing Together the Risk of Sudden Cardiac Death on Dialysis.J Am Soc Nephrol. 2019 Apr;30(4):521-523. doi: 10.1681/ASN.2019020185. Epub 2019 Mar 18. J Am Soc Nephrol. 2019. PMID: 30885936 Free PMC article. No abstract available.
-
The Case for Selective Withdrawal of Antidepressants in Patients with Advanced Kidney Disease.J Am Soc Nephrol. 2019 Jul;30(7):1339-1340. doi: 10.1681/ASN.2019030308. Epub 2019 Jun 3. J Am Soc Nephrol. 2019. PMID: 31160515 Free PMC article. No abstract available.
-
Authors' Reply.J Am Soc Nephrol. 2019 Jul;30(7):1340. doi: 10.1681/ASN.2019040431. Epub 2019 Jun 3. J Am Soc Nephrol. 2019. PMID: 31160516 Free PMC article. No abstract available.
Similar articles
-
The modifying effect of the serum-to-dialysate potassium gradient on the cardiovascular safety of SSRIs in the hemodialysis population: a pharmacoepidemiologic study.Nephrol Dial Transplant. 2022 Oct 19;37(11):2241-2252. doi: 10.1093/ndt/gfac214. Nephrol Dial Transplant. 2022. PMID: 35793567 Free PMC article.
-
Proton pump inhibitors may enhance the risk of citalopram- and escitalopram-associated sudden cardiac death among patients receiving hemodialysis.Pharmacoepidemiol Drug Saf. 2022 Jun;31(6):670-679. doi: 10.1002/pds.5428. Epub 2022 Mar 24. Pharmacoepidemiol Drug Saf. 2022. PMID: 35285107 Free PMC article.
-
A comparative study of QT prolongation with serotonin reuptake inhibitors.Psychopharmacology (Berl). 2017 Oct;234(20):3075-3081. doi: 10.1007/s00213-017-4685-7. Epub 2017 Aug 3. Psychopharmacology (Berl). 2017. PMID: 28770276
-
Use of selective serotonin reuptake inhibitors during pregnancy and risk of major and cardiovascular malformations: an update.Postgrad Med. 2010 Jul;122(4):49-65. doi: 10.3810/pgm.2010.07.2175. Postgrad Med. 2010. PMID: 20675971 Review.
-
A comparison of the risk of QT prolongation among SSRIs.Ann Pharmacother. 2013 Oct;47(10):1330-41. doi: 10.1177/1060028013501994. Epub 2013 Oct 21. Ann Pharmacother. 2013. PMID: 24259697 Review.
Cited by
-
The Risk of Ventricular Dysrhythmia or Sudden Death in Patients Receiving Serotonin Reuptake Inhibitors With Methadone: A Population-Based Study.Front Pharmacol. 2022 Apr 20;13:861953. doi: 10.3389/fphar.2022.861953. eCollection 2022. Front Pharmacol. 2022. PMID: 35517813 Free PMC article.
-
Reply to 'Depression and clinical outcomes in CKD: do anti-depressants play a role? (EQUAL Study)'.Clin Kidney J. 2022 Mar 16;15(8):1630-1632. doi: 10.1093/ckj/sfac081. eCollection 2022 Aug. Clin Kidney J. 2022. PMID: 35892026 Free PMC article. No abstract available.
-
Disease-drug and drug-drug interaction in COVID-19: Risk and assessment.Biomed Pharmacother. 2021 Jul;139:111642. doi: 10.1016/j.biopha.2021.111642. Epub 2021 Apr 27. Biomed Pharmacother. 2021. PMID: 33940506 Free PMC article. Review.
-
Recent advances in management of COVID-19: A review.Biomed Pharmacother. 2021 Nov;143:112107. doi: 10.1016/j.biopha.2021.112107. Epub 2021 Aug 27. Biomed Pharmacother. 2021. PMID: 34488083 Free PMC article. Review.
-
Use of QT Prolonging Medications by Hemodialysis Patients and Individuals Without End-Stage Kidney Disease.J Am Heart Assoc. 2020 Jul 7;9(13):e015969. doi: 10.1161/JAHA.120.015969. Epub 2020 Jun 24. J Am Heart Assoc. 2020. PMID: 32578475 Free PMC article.
References
-
- Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. .: Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int 84: 179–191, 2013 - PubMed
-
- Centers for Medicare & Medicaid Services (CMS) End-Stage Renal Disease: (ESRD) Quality Incentive Program (QIP) Payment Year (PY) 2018 final measure technical specifications. 2016. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Inst.... Accessed September 27, 2018
-
- American Psychiatric Association: Practice guideline for the treatment of patients with major depressive disorder, Third Edition, 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/g.... Accessed September 27, 2018
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

