Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea
- PMID: 30884907
- PMCID: PMC6466033
- DOI: 10.3390/v11030266
Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea
Abstract
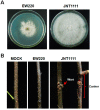
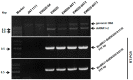
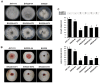
A double-stranded RNA (dsRNA) virus was isolated and characterized from strain EW220 of the phytopathogenic fungus Botryosphaeria dothidea. The full-length cDNAs of the dsRNAs were 6434 bp and 5986 bp in size, respectively. The largest dsRNA encodes a cap-pol fusion protein that contains a coat protein gene and an RNA-dependent RNA polymerase (RdRp) domain, and the second dsRNA encodes a hypothetical protein. Genome sequence analysis revealed that the sequences of the dsRNA virus shared 99% identity with Bipolaris maydis botybirnavirus 1(BmBRV1) isolated from the causal agent of corn southern leaf blight, Bipolaris maydis. Hence, the dsRNA virus constitutes a new strain of BmBRV1 and was named Bipolaris maydis botybirnavirus 1 strain BdEW220 (BmBRV1-BdEW220). BmBRV1-BdEW220 contains spherical virions that are 37 nm in diameter and consist of two dsRNA segments. The structural proteins of the BmBRV1-BdEW220 virus particles were 110 kDa, 90 kDa, and 80 kDa and were encoded by dsRNA1 and 2-ORFs. Phylogenetic reconstruction indicated that BmBRV1 and BmBRV1-BdEW220 are phylogenetically related to the genus Botybirnavirus. Importantly, BmBRV1-BdEW220 influences the growth of B. dothidea and confers hypovirulence to the fungal host. To our knowledge, this is the first report of a botybirnavirus in B. dothidea.
Keywords: Botryosphaeria dothidea; Botybirnavirus; double-stranded RNA virus; genome; hypovirulence.
Conflict of interest statement
The authors declare that they do not have any conflict of interest.
Figures



Similar articles
-
The complete genomic sequence of a novel botybirnavirus isolated from a phytopathogenic Bipolaris maydis.Virus Genes. 2018 Oct;54(5):733-736. doi: 10.1007/s11262-018-1584-x. Epub 2018 Jul 2. Virus Genes. 2018. PMID: 29967958
-
Characterization of a novel double-stranded RNA mycovirus conferring hypovirulence from the phytopathogenic fungus Botryosphaeria dothidea.Virology. 2016 Jun;493:75-85. doi: 10.1016/j.virol.2016.03.012. Epub 2016 Mar 22. Virology. 2016. PMID: 27015523
-
Co-infection of a hypovirulent isolate of Sclerotinia sclerotiorum with a new botybirnavirus and a strain of a mitovirus.Virol J. 2016 Jun 6;13:92. doi: 10.1186/s12985-016-0550-2. Virol J. 2016. PMID: 27267756 Free PMC article.
-
A neo-virus lifestyle exhibited by a (+)ssRNA virus hosted in an unrelated dsRNA virus: Taxonomic and evolutionary considerations.Virus Res. 2018 Jan 15;244:75-83. doi: 10.1016/j.virusres.2017.11.006. Epub 2017 Nov 6. Virus Res. 2018. PMID: 29122644 Review.
-
Mycovirus Diversity and Evolution Revealed/Inferred from Recent Studies.Annu Rev Phytopathol. 2022 Aug 26;60:307-336. doi: 10.1146/annurev-phyto-021621-122122. Epub 2022 May 24. Annu Rev Phytopathol. 2022. PMID: 35609970 Review.
Cited by
-
Novel Mycoviruses Discovered from a Metatranscriptomics Survey of the Phytopathogenic Alternaria Fungus.Viruses. 2022 Nov 18;14(11):2552. doi: 10.3390/v14112552. Viruses. 2022. PMID: 36423161 Free PMC article.
-
Mycovirus-induced hypovirulence in notorious fungi Sclerotinia: a comprehensive review.Braz J Microbiol. 2023 Sep;54(3):1459-1478. doi: 10.1007/s42770-023-01073-4. Epub 2023 Jul 31. Braz J Microbiol. 2023. PMID: 37523037 Free PMC article. Review.
-
Why Do We Need Alternative Methods for Fungal Disease Management in Plants?Plants (Basel). 2023 Nov 10;12(22):3822. doi: 10.3390/plants12223822. Plants (Basel). 2023. PMID: 38005718 Free PMC article. Review.
-
Molecular characterization of a novel narnavirus infecting the phytopathogenic fungus Botryosphaeria dothidea.Arch Virol. 2024 Feb 1;169(2):38. doi: 10.1007/s00705-024-05964-1. Arch Virol. 2024. PMID: 38300296
-
Characterisation of the RNA Virome of Nine Ochlerotatus Species in Finland.Viruses. 2022 Jul 7;14(7):1489. doi: 10.3390/v14071489. Viruses. 2022. PMID: 35891469 Free PMC article.
References
-
- Donaire L., Pagán I., Ayllón M.A. Characterization of Botrytis cinerea negative-stranded RNA virus 1, a new mycovirus related to plant viruses, and a reconstruction of host pattern evolution in negative-sense ssRNA viruses. Virology. 2016;499:212–218. doi: 10.1016/j.virol.2016.09.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

