Reliability of Measures Intended to Assess Threshold-Independent Hearing Disorders
- PMID: 30882533
- PMCID: PMC6745005
- DOI: 10.1097/AUD.0000000000000711
Reliability of Measures Intended to Assess Threshold-Independent Hearing Disorders
Abstract
Objectives: Recent animal studies have shown that noise exposure can cause cochlear synaptopathy without permanent threshold shift. Because the noise exposure preferentially damaged auditory nerve fibers that processed suprathreshold sounds (low-spontaneous rate fibers), it has been suggested that synaptopathy may underlie suprathreshold hearing deficits in humans. Recently, several researchers have suggested measures to identify the pathology or pathologies underlying suprathreshold hearing deficits in humans based on results from animal studies; however, the reliability of some of these measures have not been assessed. The purpose of this study was to assess the test-retest reliability of measures that may have the potential to relate suprathreshold hearing deficits to site(s)-of-lesion along the peripheral auditory system in humans.
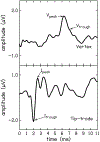
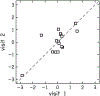
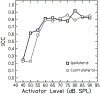
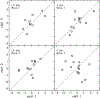
Design: Adults with audiometric normal hearing were tested on a battery of behavioral and physiologic measures that included (1) thresholds in quiet (TIQ), (2) thresholds in noise (TIN), (3) frequency-modulation detection threshold (FMDT), (4) word recognition in four listening conditions, (5) distortion-product otoacoustic emissions (DPOAE), (6) middle ear muscle reflex (MEMR), (7) tone burst-elicited auditory brainstem response (tbABR), and (8) speech-evoked ABR (sABR). Data collection for each measure was repeated over two visits separated by at least one week. The residuals of the correlation between the suprathreshold measures and TIQ serve as functional and quantitative proxies for threshold-independent hearing disorders because they represent the portion of the raw measures that is not dependent on TIQ. Reliability of the residual measures was assessed using intraclass correlation (ICC).
Results: Reliability for the residual measures was good (ICC ≥ 0.75) for FMDT, DPOAEs, and MEMR. Residual measures showing moderate reliability (0.5 ≤ ICC < 0.75) were tbABR wave I amplitude, TIN, and word recognition in quiet, noise, and time-compressed speech with reverberation. Wave V of the tbABR, waves of the sABR, and recognition of time-compressed words had poor test-retest reliability (ICC < 0.5).
Conclusions: Reliability of residual measures was mixed, suggesting that care should be taken when selecting measures for diagnostic tests of threshold-independent hearing disorders. Quantifying hidden hearing loss as the variance in suprathreshold measures of auditory function that is not due to TIQ may provide a reliable estimate of threshold-independent hearing disorders in humans.
Figures



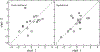

Similar articles
-
Middle Ear Muscle Reflex and Word Recognition in "Normal-Hearing" Adults: Evidence for Cochlear Synaptopathy?Ear Hear. 2020 Jan/Feb;41(1):25-38. doi: 10.1097/AUD.0000000000000804. Ear Hear. 2020. PMID: 31584501 Free PMC article.
-
Using Thresholds in Noise to Identify Hidden Hearing Loss in Humans.Ear Hear. 2018 Sep/Oct;39(5):829-844. doi: 10.1097/AUD.0000000000000543. Ear Hear. 2018. PMID: 29337760 Free PMC article.
-
Suprathreshold Auditory Measures for Detecting Early-Stage Noise-Induced Hearing Loss in Young Adults.J Am Acad Audiol. 2022 Apr;33(4):185-195. doi: 10.1055/s-0041-1740362. Epub 2022 Oct 4. J Am Acad Audiol. 2022. PMID: 36195294 Free PMC article.
-
Effects of Recreational Noise on Threshold and Suprathreshold Measures of Auditory Function.Semin Hear. 2017 Nov;38(4):298-318. doi: 10.1055/s-0037-1606325. Epub 2017 Oct 10. Semin Hear. 2017. PMID: 29026263 Free PMC article. Review.
-
[Otoacoustic emissions, auditory evoked potentials, pure tone thresholds and speech intelligibility in cases of auditory neuropathy].HNO. 2000 Jan;48(1):28-32. doi: 10.1007/s001060050005. HNO. 2000. PMID: 10663046 Review. German.
Cited by
-
Animal-to-Human Translation Difficulties and Problems With Proposed Coding-in-Noise Deficits in Noise-Induced Synaptopathy and Hidden Hearing Loss.Front Neurosci. 2022 May 23;16:893542. doi: 10.3389/fnins.2022.893542. eCollection 2022. Front Neurosci. 2022. PMID: 35720689 Free PMC article. Review.
-
Evidence for Loss of Activity in Low-Spontaneous-Rate Auditory Nerve Fibers of Older Adults.J Assoc Res Otolaryngol. 2022 Apr;23(2):273-284. doi: 10.1007/s10162-021-00827-x. Epub 2022 Jan 12. J Assoc Res Otolaryngol. 2022. PMID: 35020090 Free PMC article.
-
Prevention of Noise-Induced Hearing Loss Using Investigational Medicines for the Inner Ear: Previous Trial Outcomes Should Inform Future Trial Design.Antioxid Redox Signal. 2022 Jun;36(16-18):1171-1202. doi: 10.1089/ars.2021.0166. Epub 2021 Oct 4. Antioxid Redox Signal. 2022. PMID: 34346254 Free PMC article. Review.
-
Middle Ear Muscle Reflex in Normal-Hearing Individuals with Occupational Noise Exposure.Noise Health. 2023 Jan-Mar;25(116):1-7. doi: 10.4103/nah.nah_3_22. Noise Health. 2023. PMID: 37006113 Free PMC article.
-
The Rise and Fall of Aural Acoustic Immittance Assessment Tools.Semin Hear. 2023 Mar 14;44(1):5-16. doi: 10.1055/s-0043-1764139. eCollection 2023 Feb. Semin Hear. 2023. PMID: 36925655 Free PMC article. Review.
References
-
- Akhoun I, Gallégo S, Moulin A, Ménard M, Veuillet E, Berger-Vachon C, … Thai-Van H (2008). The temporal relationship between speech auditory brainstem responses and the acoustic pattern of the phoneme /ba/ in normal-hearing adults. Clinical Neurophysiology, 119, 922–933. 10.1016/j.clinph.2007.12.010 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

