Characterization of a novel cathepsin L-like protease from Taenia solium metacestodes for the immunodiagnosis of porcine cysticercosis
- PMID: 30878092
- PMCID: PMC7010362
- DOI: 10.1016/j.vetpar.2019.01.004
Characterization of a novel cathepsin L-like protease from Taenia solium metacestodes for the immunodiagnosis of porcine cysticercosis
Abstract
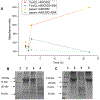
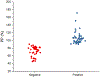
Porcine cysticercosis is an endemic parasitic disease caused by infection with Taenia solium that is found predominantly in developing countries. In order to aid in the development of simple diagnostic approaches, identification and characterization of potential new antigens for immunodiagnostic purposes is desired. The cysteine protease family has previously been found to have important immunodiagnostic properties. These proteases are expressed as zymogens which contain a signal peptide, pro-peptide, and an active domain. Subsequent catalytic cleavage of the pro-peptide converts these zymogens into enzymes. With the use of bioinformatic tools we identified an active domain of a novel cathepsin L-like cysteine protease (TsolCL) in the T. solium genome. The TsolCL gene includes 705 nucleotides (nt) within a single intron and a 633 nt exonic sequence encoding an active protein of 211 amino acids. Sequence alignment and phylogenetic analysis suggest that the TsolCL gene is closely related to genes found in Echinoccocus granulosus and E. multiloculars. In addition, TsolCL was found to have a 61.9%-99.0% similarity to other cathepsin L proteins found in other helminths and mammals. We cloned, expressed, purified, and characterized the recombinant active TsolCL (27 kDa) using the baculovirus-insect cell expression system. TsolCL showed cysteine protease enzymatic activity with the capacity to hydrolyze the Z-Phe-Arg-AMC substrate as well as bovine serum albumin. However, TsolCL was not able to hydrolyze human immunoglobulin. In addition, TsolCL has cathepsin L conserved amino acid residues in the catalytic site (Gln8, Cys14, His159, Asn179 and Trp181) and the motif GCNGG. Using ELISA, TsolCL was able to distinguish circulating IgG antibodies between healthy animals and naturally infected pigs with cysticercosis, showing a moderate sensitivity of 83.33% (40/48; 95% CI: [69.8%-92.5 %]), and a specificity of 83.78% (31/37; 95% CI: [67.9%-93.8%]). In conclusion, a novel cathepsin L-like cysteine protease from a T. solium metacestode was expressed successfully in Baculovirus system and was evaluated as a candidate antigen to diagnose porcine cysticercosis using the ELISA immunoassay.
Keywords: Baculovirus expression vector system; Cathepsin L; Cysticercosis; Immunodiagnosis; Taenia solium.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


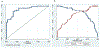
Similar articles
-
A novel enolase from Taenia solium metacestodes and its evaluation as an immunodiagnostic antigen for porcine cysticercosis.Exp Parasitol. 2018 Aug;191:44-54. doi: 10.1016/j.exppara.2018.06.001. Epub 2018 Jun 7. Exp Parasitol. 2018. PMID: 29885292
-
TsPKA-r: a potential immunodiagnostic antigen for the detection of porcine cysticercosis.Acta Trop. 2017 Jul;171:80-85. doi: 10.1016/j.actatropica.2017.03.026. Epub 2017 Mar 28. Acta Trop. 2017. PMID: 28359828
-
Evaluation of recombinant HP6-Tsag, an 18 kDa Taenia saginata oncospheral adhesion protein, for the diagnosis of cysticercosis.Parasitol Res. 2007 Aug;101(3):517-25. doi: 10.1007/s00436-007-0507-x. Epub 2007 Mar 12. Parasitol Res. 2007. PMID: 17351832
-
Immunodiagnostic tools for human and porcine cysticercosis.Acta Trop. 2003 Jun;87(1):79-86. doi: 10.1016/s0001-706x(03)00058-5. Acta Trop. 2003. PMID: 12781381 Review.
-
[Cathepsin L cysteine protease from Taenia solium: its biological role in the infection and potential use for the immunodiagnosis of neurocysticercosis].Rev Peru Med Exp Salud Publica. 2013 Jul;30(3):446-54. Rev Peru Med Exp Salud Publica. 2013. PMID: 24100821 Review. Spanish.
Cited by
-
Taenia solium Cysticercosis and Its Impact in Neurological Disease.Clin Microbiol Rev. 2020 May 27;33(3):e00085-19. doi: 10.1128/CMR.00085-19. Print 2020 Jun 17. Clin Microbiol Rev. 2020. PMID: 32461308 Free PMC article. Review.
-
Molecular characterization of EcCLP1, a new putative cathepsin L protease from Echinococcus canadensis.Parasite. 2024;31:39. doi: 10.1051/parasite/2024036. Epub 2024 Jul 9. Parasite. 2024. PMID: 38995112 Free PMC article.
-
Molecular characterization and determination of the biochemical properties of cathepsin L of Trichinella spiralis.Vet Res. 2022 Jun 23;53(1):48. doi: 10.1186/s13567-022-01065-6. Vet Res. 2022. PMID: 35739604 Free PMC article.
References
-
- Brooks SA, 2004. Appropriate glycosylation of recombinant proteins for human use: implications of choice of expression system. Mol. Biotechnol 28, 241–255. - PubMed
-
- Conraths FJ, Deplazes P, 2015. Echinococcus multilocularis: Epidemiology, surveillance and state-of-the-art diagnostics from a veterinary public health perspective. Vet. Parasitol 213, 149–161. - PubMed
-
- Cortez AP, Rodrigues AC, Garcia HA, Neves L, Batista JS, Bengaly Z, Paiva F, Teixeira MMG, 2009. Cathepsin L-like genes of Trypanosoma vivax from Africa and South America – characterization, relationships and diagnostic implications. Mol. Cell. Probes 23, 44–51. - PubMed
-
- Demain AL, Vaishnav P, 2009. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv 27, 297–306. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

