Carbocisteine Improves Histone Deacetylase 2 Deacetylation Activity via Regulating Sumoylation of Histone Deacetylase 2 in Human Tracheobronchial Epithelial Cells
- PMID: 30873037
- PMCID: PMC6400890
- DOI: 10.3389/fphar.2019.00166
Carbocisteine Improves Histone Deacetylase 2 Deacetylation Activity via Regulating Sumoylation of Histone Deacetylase 2 in Human Tracheobronchial Epithelial Cells
Abstract
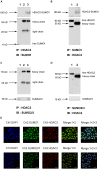
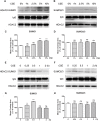
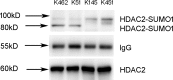
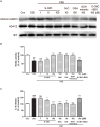
Histone deacetylase (HDAC) 2 plays a vital role in modifying histones to mediate inflammatory responses, while HDAC2 itself is commonly regulated by post-translational modifications. Small ubiquitin-related modifier (SUMO), as an important PTM factor, is involved in the regulation of multiple protein functions. Our previous studies have shown that carbocisteine (S-CMC) reversed cigarette smoke extract (CSE)-induced down-regulation of HDAC2 expression/activity in a thiol/GSH-dependent manner and enhanced sensitivity of steroid therapy. However, the mechanism by which S-CMC regulates HDAC2 is worth further exploring. Our study aimed to investigate the relationships between HDAC2 sumoylation and its deacetylase activity under oxidative stress and the molecular mechanism of S-CMC to regulate HDAC2 activity that mediates inflammatory responses in human bronchial epithelial cells. We found that modification of HDAC2 by SUMO1 and SUMO2/3 occurred in 16HBE cells under physiological conditions, and CSE induced SUMO1 modification of HDAC2 in a dose and time-dependent manner. K462 and K51 of HDAC2 were the two major modification sites of SUMO1, and the K51 site mediated deacetylation activity and function of HDAC2 on histone H4 that regulates IL-8 secretion. S-CMC inhibited CSE-induced SUMO1 modification of HDAC2 in the presence of thiol/GSH, increased HDAC activity, and decreased IL-8 expression. Our study may provide novel mechanistic explanation of S-CMC to ameliorate steroid sensitivity treatment in chronic obstructive pulmonary disease.
Keywords: GSH/thiol; carbocisteine; cigarette smoke extract; histone deacetylase 2; small ubiquitin-related modifier.
Figures

Similar articles
-
Carbocysteine restores steroid sensitivity by targeting histone deacetylase 2 in a thiol/GSH-dependent manner.Pharmacol Res. 2015 Jan;91:88-98. doi: 10.1016/j.phrs.2014.12.002. Epub 2014 Dec 11. Pharmacol Res. 2015. PMID: 25500537
-
Detection of Sumo Modification of Endogenous Histone Deacetylase 2 (HDAC2) in Mammalian Cells.Methods Mol Biol. 2016;1436:15-22. doi: 10.1007/978-1-4939-3667-0_2. Methods Mol Biol. 2016. PMID: 27246205
-
A mucoactive drug carbocisteine ameliorates steroid resistance in rat COPD model.Pulm Pharmacol Ther. 2016 Aug;39:38-47. doi: 10.1016/j.pupt.2016.06.003. Epub 2016 Jun 18. Pulm Pharmacol Ther. 2016. PMID: 27328977
-
Histone deacetylation: an important mechanism in inflammatory lung diseases.COPD. 2005 Dec;2(4):445-55. doi: 10.1080/15412550500346683. COPD. 2005. PMID: 17147010 Review.
-
Histone deacetylase 2 controls p53 and is a critical factor in tumorigenesis.Biochim Biophys Acta. 2014 Dec;1846(2):524-38. doi: 10.1016/j.bbcan.2014.07.010. Epub 2014 Jul 27. Biochim Biophys Acta. 2014. PMID: 25072962 Review.
Cited by
-
The role of sulfur compounds in chronic obstructive pulmonary disease.Front Mol Biosci. 2022 Oct 19;9:928287. doi: 10.3389/fmolb.2022.928287. eCollection 2022. Front Mol Biosci. 2022. PMID: 36339716 Free PMC article. Review.
-
Cellular and Molecular Signatures of Oxidative Stress in Bronchial Epithelial Cell Models Injured by Cigarette Smoke Extract.Int J Mol Sci. 2022 Feb 4;23(3):1770. doi: 10.3390/ijms23031770. Int J Mol Sci. 2022. PMID: 35163691 Free PMC article. Review.
-
Arabidopsis SUMO E3 Ligase SIZ1 Interacts with HDA6 and Negatively Regulates HDA6 Function during Flowering.Cells. 2021 Nov 3;10(11):3001. doi: 10.3390/cells10113001. Cells. 2021. PMID: 34831226 Free PMC article.
-
The emerging roles of SUMOylation in pulmonary diseases.Mol Med. 2023 Sep 5;29(1):119. doi: 10.1186/s10020-023-00719-1. Mol Med. 2023. PMID: 37670258 Free PMC article. Review.
-
The Current Molecular and Cellular Landscape of Chronic Obstructive Pulmonary Disease (COPD): A Review of Therapies and Efforts towards Personalized Treatment.Proteomes. 2024 Aug 16;12(3):23. doi: 10.3390/proteomes12030023. Proteomes. 2024. PMID: 39189263 Free PMC article. Review.
References
-
- Bahnassy S., Kumar S., Ren J., Frutiz G., Karami S., Bawa-Khalfe T. (2017). Androgen receptor in tamoxifen-resistant breast cancer is affected by SUMO. Cancer. Res 77(4 Suppl.):P3-04–21.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

