Reciprocal modulation of 5-HT and octopamine regulates pumping via feedforward and feedback circuits in C. elegans
- PMID: 30872487
- PMCID: PMC6452730
- DOI: 10.1073/pnas.1819261116
Reciprocal modulation of 5-HT and octopamine regulates pumping via feedforward and feedback circuits in C. elegans
Erratum in
-
Correction for Liu et al., Reciprocal modulation of 5-HT and octopamine regulates pumping via feedforward and feedback circuits in C. elegans.Proc Natl Acad Sci U S A. 2019 May 21;116(21):10598. doi: 10.1073/pnas.1906704116. Epub 2019 May 13. Proc Natl Acad Sci U S A. 2019. PMID: 31085634 Free PMC article. No abstract available.
Abstract
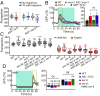
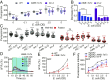
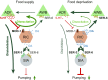
Feeding is vital for animal survival and is tightly regulated by the endocrine and nervous systems. To study the mechanisms of humoral regulation of feeding behavior, we investigated serotonin (5-HT) and octopamine (OA) signaling in Caenorhabditis elegans, which uses pharyngeal pumping to ingest bacteria into the gut. We reveal that a cross-modulation mechanism between 5-HT and OA, which convey feeding and fasting signals, respectively, mainly functions in regulating the pumping and secretion of both neuromodulators via ADF/RIC/SIA feedforward neurocircuit (consisting of ADF, RIC, and SIA neurons) and ADF/RIC/AWB/ADF feedback neurocircuit (consisting of ADF, RIC, AWB, and ADF neurons) under conditions of food supply and food deprivation, respectively. Food supply stimulates food-sensing ADFs to release more 5-HT, which augments pumping via inhibiting OA secretion by RIC interneurons and, thus, alleviates pumping suppression by OA-activated SIA interneurons/motoneurons. In contrast, nutrient deprivation stimulates RICs to secrete OA, which suppresses pumping via activating SIAs and maintains basal pumping and 5-HT production activity through excitation of ADFs relayed by AWB sensory neurons. Notably, the feedforward and feedback circuits employ distinct modalities of neurosignal integration, namely, disinhibition and disexcitation, respectively.
Keywords: C. elegans; disexcitation; octopamine; pharyngeal pumping; serotonin.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Antagonistic Serotonergic and Octopaminergic Neural Circuits Mediate Food-Dependent Locomotory Behavior in Caenorhabditis elegans.J Neurosci. 2017 Aug 16;37(33):7811-7823. doi: 10.1523/JNEUROSCI.2636-16.2017. Epub 2017 Jul 11. J Neurosci. 2017. PMID: 28698386 Free PMC article.
-
Reciprocal inhibition between sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans.Nat Commun. 2015 Jan 13;6:5655. doi: 10.1038/ncomms6655. Nat Commun. 2015. PMID: 25585042
-
Serotonin regulates repolarization of the C. elegans pharyngeal muscle.J Exp Biol. 2003 Jan;206(Pt 2):223-31. doi: 10.1242/jeb.00101. J Exp Biol. 2003. PMID: 12477893 Free PMC article.
-
The regulation of feeding and metabolism in response to food deprivation in Caenorhabditis elegans.Invert Neurosci. 2010 Dec;10(2):63-76. doi: 10.1007/s10158-010-0112-z. Epub 2010 Dec 1. Invert Neurosci. 2010. PMID: 21120572 Review.
-
Serotonergic modulation of feeding behavior in Caenorhabditis elegans and other related nematodes.Neurosci Res. 2020 May;154:9-19. doi: 10.1016/j.neures.2019.04.006. Epub 2019 Apr 24. Neurosci Res. 2020. PMID: 31028772 Review.
Cited by
-
Positive interaction between ASH and ASK sensory neurons accelerates nociception and inhibits behavioral adaptation.iScience. 2022 Oct 6;25(11):105287. doi: 10.1016/j.isci.2022.105287. eCollection 2022 Nov 18. iScience. 2022. PMID: 36304123 Free PMC article.
-
Serotonergic modulation across sensory modalities.J Neurophysiol. 2020 Jun 1;123(6):2406-2425. doi: 10.1152/jn.00034.2020. Epub 2020 May 13. J Neurophysiol. 2020. PMID: 32401124 Free PMC article. Review.
-
Pongamol Prevents Neurotoxicity via the Activation of MAPKs/Nrf2 Signaling Pathway in H2O2-Induced Neuronal PC12 Cells and Prolongs the Lifespan of Caenorhabditis elegans.Mol Neurobiol. 2024 Oct;61(10):8219-8233. doi: 10.1007/s12035-024-04110-x. Epub 2024 Mar 14. Mol Neurobiol. 2024. PMID: 38483657
-
Molecular and circuit mechanisms underlying avoidance of rapid cooling stimuli in C. elegans.Nat Commun. 2024 Jan 5;15(1):297. doi: 10.1038/s41467-023-44638-5. Nat Commun. 2024. PMID: 38182628 Free PMC article.
-
Methods to extract and study the biological effects of murine gut microbiota using Caenorhabditis elegans as a screening host.PLoS One. 2023 Feb 23;18(2):e0281887. doi: 10.1371/journal.pone.0281887. eCollection 2023. PLoS One. 2023. PMID: 36821579 Free PMC article.
References
-
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. - PubMed
-
- Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol. 2010;6:255–269. - PubMed
-
- Lemieux GA, Ashrafi K. Neural regulatory pathways of feeding and fat in Caenorhabditis elegans. Annu Rev Genet. 2015;49:413–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

