Slicing and dicing viruses: antiviral RNA interference in mammals
- PMID: 30872283
- PMCID: PMC6463209
- DOI: 10.15252/embj.2018100941
Slicing and dicing viruses: antiviral RNA interference in mammals
Abstract
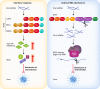
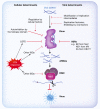
To protect against the harmful consequences of viral infections, organisms are equipped with sophisticated antiviral mechanisms, including cell-intrinsic means to restrict viral replication and propagation. Plant and invertebrate cells utilise mostly RNA interference (RNAi), an RNA-based mechanism, for cell-intrinsic immunity to viruses while vertebrates rely on the protein-based interferon (IFN)-driven innate immune system for the same purpose. The RNAi machinery is conserved in vertebrate cells, yet whether antiviral RNAi is still active in mammals and functionally relevant to mammalian antiviral defence is intensely debated. Here, we discuss cellular and viral factors that impact on antiviral RNAi and the contexts in which this system might be at play in mammalian resistance to viral infection.
Keywords: Dicer; RNA interference; antiviral immunity; double‐stranded RNA; interferons.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Antiviral innate immune response of RNA interference.J Infect Dev Ctries. 2014 Jul 14;8(7):804-10. doi: 10.3855/jidc.4187. J Infect Dev Ctries. 2014. PMID: 25022288 Review.
-
Antiviral RNA interference in mammals.Curr Opin Immunol. 2018 Oct;54:109-114. doi: 10.1016/j.coi.2018.06.010. Epub 2018 Jul 17. Curr Opin Immunol. 2018. PMID: 30015086 Free PMC article. Review.
-
RNAi-mediated antiviral immunity in mammals.Curr Opin Virol. 2018 Oct;32:9-14. doi: 10.1016/j.coviro.2018.07.008. Epub 2018 Jul 14. Curr Opin Virol. 2018. PMID: 30015014 Review.
-
The silent treatment: RNAi as a defense against virus infection in mammals.Trends Biotechnol. 2006 Apr;24(4):186-93. doi: 10.1016/j.tibtech.2006.02.006. Epub 2006 Feb 28. Trends Biotechnol. 2006. PMID: 16503061 Review.
-
Processing of double-stranded RNA in mammalian cells: a direct antiviral role?J Interferon Cytokine Res. 2014 Jun;34(6):469-77. doi: 10.1089/jir.2014.0003. J Interferon Cytokine Res. 2014. PMID: 24905204 Review.
Cited by
-
RNAi suppressor: The hidden weapon of SARS-CoV.J Biosci. 2020;45(1):99. doi: 10.1007/s12038-020-00071-0. J Biosci. 2020. PMID: 32713862 Free PMC article. Review.
-
SARS-CoV-2 RNAs are processed into 22-nt vsRNAs in Vero cells.Front Immunol. 2022 Oct 28;13:1008084. doi: 10.3389/fimmu.2022.1008084. eCollection 2022. Front Immunol. 2022. PMID: 36389816 Free PMC article.
-
The SARS-CoV-2 targeted human RNA binding proteins network biology to investigate COVID-19 associated manifestations.Int J Biol Macromol. 2022 Sep 30;217:853-863. doi: 10.1016/j.ijbiomac.2022.07.200. Epub 2022 Jul 28. Int J Biol Macromol. 2022. PMID: 35907451 Free PMC article.
-
Dicer and PKR as Novel Regulators of Embryonic Stem Cell Fate and Antiviral Innate Immunity.J Immunol. 2022 May 15;208(10):2259-2266. doi: 10.4049/jimmunol.2200042. J Immunol. 2022. PMID: 35577384 Free PMC article. Review.
-
Enhanced expression of the human Survival motor neuron 1 gene from a codon-optimised cDNA transgene in vitro and in vivo.Gene Ther. 2023 Dec;30(12):812-825. doi: 10.1038/s41434-023-00406-0. Epub 2023 Jun 15. Gene Ther. 2023. PMID: 37322133
References
-
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

