Adhesion G Protein-Coupled Receptors as Drug Targets for Neurological Diseases
- PMID: 30871735
- PMCID: PMC6433515
- DOI: 10.1016/j.tips.2019.02.003
Adhesion G Protein-Coupled Receptors as Drug Targets for Neurological Diseases
Abstract
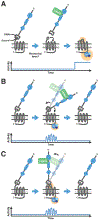
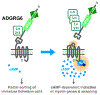
The family of adhesion G protein-coupled receptors (aGPCRs) consists of 33 members in humans. Although the majority are orphan receptors with unknown functions, many reports have demonstrated critical functions for some members of this family in organogenesis, neurodevelopment, myelination, angiogenesis, and cancer progression. Importantly, mutations in several aGPCRs have been linked to human diseases. The crystal structure of a shared protein domain, the GPCR Autoproteolysis INducing (GAIN) domain, has enabled the discovery of a common signaling mechanism - a tethered agonist - for this class of receptors. A series of recent reports has shed new light on their biological functions and disease relevance. This review focuses on these recent advances in our understanding of aGPCR biology in the nervous system and the untapped potential of aGPCRs as novel therapeutic targets for neurological disease.
Keywords: GAIN domain; GPCR Autoproteolysis INducing domain; adhesion G protein-coupled receptors; neurodevelopment; neurological disease.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Adhesion G protein-coupled receptor gluing action guides tissue development and disease.J Mol Med (Berl). 2022 Oct;100(10):1355-1372. doi: 10.1007/s00109-022-02240-0. Epub 2022 Aug 15. J Mol Med (Berl). 2022. PMID: 35969283 Review.
-
Mechanisms of adhesion G protein-coupled receptor activation.J Biol Chem. 2020 Oct 9;295(41):14065-14083. doi: 10.1074/jbc.REV120.007423. Epub 2020 Aug 6. J Biol Chem. 2020. PMID: 32763969 Free PMC article. Review.
-
7TM Domain Structure of Adhesion GPCRs.Handb Exp Pharmacol. 2016;234:43-66. doi: 10.1007/978-3-319-41523-9_3. Handb Exp Pharmacol. 2016. PMID: 27832483 Review.
-
Adhesion G Protein-Coupled Receptors as Drug Targets.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:429-449. doi: 10.1146/annurev-pharmtox-010617-052933. Epub 2017 Oct 2. Annu Rev Pharmacol Toxicol. 2018. PMID: 28968187 Free PMC article. Review.
-
Adhesion G protein-coupled receptors: opportunities for drug discovery.Nat Rev Drug Discov. 2019 Nov;18(11):869-884. doi: 10.1038/s41573-019-0039-y. Epub 2019 Aug 28. Nat Rev Drug Discov. 2019. PMID: 31462748
Cited by
-
Regulation of pulmonary surfactant by the adhesion GPCR GPR116/ADGRF5 requires a tethered agonist-mediated activation mechanism.Elife. 2022 Sep 8;11:e69061. doi: 10.7554/eLife.69061. Elife. 2022. PMID: 36073784 Free PMC article.
-
Optimization of a peptide ligand for the adhesion GPCR ADGRG2 provides a potent tool to explore receptor biology.J Biol Chem. 2021 Jan-Jun;296:100174. doi: 10.1074/jbc.RA120.014726. Epub 2020 Dec 17. J Biol Chem. 2021. PMID: 33303626 Free PMC article.
-
Adhesion G protein-coupled receptors: structure, signaling, physiology, and pathophysiology.Physiol Rev. 2022 Oct 1;102(4):1587-1624. doi: 10.1152/physrev.00027.2021. Epub 2022 Apr 25. Physiol Rev. 2022. PMID: 35468004 Free PMC article. Review.
-
The adhesion GPCRs CELSR1-3 and LPHN3 engage G proteins via distinct activation mechanisms.Cell Rep. 2023 Jun 27;42(6):112552. doi: 10.1016/j.celrep.2023.112552. Epub 2023 May 23. Cell Rep. 2023. PMID: 37224017 Free PMC article.
-
G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders.Signal Transduct Target Ther. 2023 May 3;8(1):177. doi: 10.1038/s41392-023-01427-2. Signal Transduct Target Ther. 2023. PMID: 37137892 Free PMC article. Review.
References
-
- Bjarnadottir TK, et al., The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics, 2004. 84(1): p. 23–33. - PubMed

