Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review
- PMID: 30871502
- PMCID: PMC6419460
- DOI: 10.1186/s12874-019-0695-y
Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review
Abstract
Background: The availability of clinical and therapeutic data drawn from medical records and administrative databases has entailed new opportunities for clinical and epidemiologic research. However, these databases present inherent limitations which may render them prone to new biases. We aimed to conduct a structured review of biases specific to observational clinical studies based on secondary databases, and to propose strategies for the mitigation of those biases.
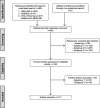
Methods: Scoping review of the scientific literature published during the period 2000-2018 through an automated search of MEDLINE, EMBASE and Web of Science, supplemented with manually cross-checking of reference lists. We included opinion essays, methodological reviews, analyses or simulation studies, as well as letters to the editor or retractions, the principal objective of which was to highlight the existence of some type of bias in pharmacoepidemiologic studies using secondary databases.
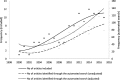
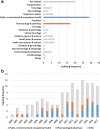
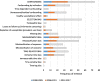
Results: A total of 117 articles were included. An increasing trend in the number of publications concerning the potential limitations of secondary databases was observed over time and across medical research disciplines. Confounding was the most reported category of bias (63.2% of articles), followed by selection and measurement biases (47.0% and 46.2% respectively). Confounding by indication (32.5%), unmeasured/residual confounding (28.2%), outcome misclassification (28.2%) and "immortal time" bias (25.6%) were the subcategories most frequently mentioned.
Conclusions: Suboptimal use of secondary databases in pharmacoepidemiologic studies has introduced biases in the studies, which may have led to erroneous conclusions. Methods to mitigate biases are available and must be considered in the design, analysis and interpretation phases of studies using these data sources.
Keywords: Administrative claims; Bias; Confounding factors; Electronic health records; Medical record linkage; Medical records; Observational studies; Pharmacoepidemiology.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Core concepts in pharmacoepidemiology: Key biases arising in pharmacoepidemiologic studies.Pharmacoepidemiol Drug Saf. 2023 Jan;32(1):9-18. doi: 10.1002/pds.5547. Epub 2022 Oct 20. Pharmacoepidemiol Drug Saf. 2023. PMID: 36216785 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Review. Italian.
-
Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations.Pharmacoepidemiol Drug Saf. 2014 Sep;23(9):891-901. doi: 10.1002/pds.3674. Epub 2014 Jun 24. Pharmacoepidemiol Drug Saf. 2014. PMID: 24962929 Free PMC article. Review.
-
Implementation of the trial emulation approach in medical research: a scoping review.BMC Med Res Methodol. 2023 Aug 16;23(1):186. doi: 10.1186/s12874-023-02000-9. BMC Med Res Methodol. 2023. PMID: 37587484 Free PMC article. Review.
Cited by
-
Dying Too Soon: Excess Mortality in Severe Mental Illness.Front Psychiatry. 2019 Dec 6;10:855. doi: 10.3389/fpsyt.2019.00855. eCollection 2019. Front Psychiatry. 2019. PMID: 31920734 Free PMC article.
-
Usage of primary and administrative data to measure the economic impact of quality improvement projects.BMJ Open Qual. 2020 Apr;9(2):e000712. doi: 10.1136/bmjoq-2019-000712. BMJ Open Qual. 2020. PMID: 32276970 Free PMC article. Review. No abstract available.
-
Association of 5α-Reductase Inhibitor Prescription With Bladder Cancer Progression in Males in South Korea.JAMA Netw Open. 2023 May 1;6(5):e2313667. doi: 10.1001/jamanetworkopen.2023.13667. JAMA Netw Open. 2023. PMID: 37191958 Free PMC article.
-
Clinical Use of Acid Suppressants and Risk of Dementia in the Elderly: A Pharmaco-Epidemiological Cohort Study.Int J Environ Res Public Health. 2020 Nov 9;17(21):8271. doi: 10.3390/ijerph17218271. Int J Environ Res Public Health. 2020. PMID: 33182362 Free PMC article.
-
Evolution of the profiles of new psychotropic drug users before and during the COVID-19 crisis: an original longitudinal approach through multichannel sequence analysis using the French health-care database.Eur Arch Psychiatry Clin Neurosci. 2024 Mar 18. doi: 10.1007/s00406-024-01774-3. Online ahead of print. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 38499795
References
-
- Hennessy S. Use of health care databases in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:311–313. - PubMed
-
- Ray WA. Improving automated database studies. Epidemiology. 2011;22:302–304. - PubMed
-
- Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. - PubMed
-
- European Commission. Regulation (EU) 2016/679 of the European Parliament and of the council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing directive 95/46/EC (general data protection regulation). 2016. http://eur-lex.europa.eu/eli/reg/2016/679/oj. Accessed 8 Oct 2018.
-
- U.S. Department of Health and Human Services. Code of Federal Regulations. Title 45 Public Welfare. Part 46 Protection of Human Subjects. 2016. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/in.... Accessed 8 Oct 2018. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

