Development of DNA Aptamers to Native EpCAM for Isolation of Lung Circulating Tumor Cells from Human Blood
- PMID: 30871104
- PMCID: PMC6468627
- DOI: 10.3390/cancers11030351
Development of DNA Aptamers to Native EpCAM for Isolation of Lung Circulating Tumor Cells from Human Blood
Abstract
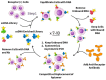
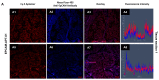
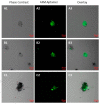
We selected DNA aptamers to the epithelial cell adhesion molecule (EpCAM) expressed on primary lung cancer cells isolated from the tumors of patients with non-small cell lung cancer using competitive displacement of aptamers from EpCAM by a corresponding antibody. The resulting aptamers clones showed good nanomolar affinity to EpCAM-positive lung cancer cells. Confocal microscopy imaging and spectral profiling of lung cancer tissues confirmed the same protein target for the aptamers and anti-EpCAM antibodies. Furthermore, the resulted aptamers were successfully applied for isolation and detection of circulating tumor cells in clinical samples of peripheral blood of lung cancer patients.
Keywords: EpCAM; SELEX (Systematic evolution of ligands by exponential enrichment); aptamers; blood; circulating tumor cells; non-small-cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture.Anal Chem. 2013 Apr 16;85(8):4141-9. doi: 10.1021/ac400366b. Epub 2013 Mar 29. Anal Chem. 2013. PMID: 23480100
-
Isolation of DNA aptamers targeting N-cadherin and high-efficiency capture of circulating tumor cells by using dual aptamers.Nanoscale. 2020 Nov 19;12(44):22574-22585. doi: 10.1039/d0nr06180h. Nanoscale. 2020. PMID: 33174555
-
Selection and targeting of EpCAM protein by ssDNA aptamer.PLoS One. 2017 Dec 15;12(12):e0189558. doi: 10.1371/journal.pone.0189558. eCollection 2017. PLoS One. 2017. PMID: 29245156 Free PMC article.
-
Antibody Based EpCAM Targeted Therapy of Cancer, Review and Update.Curr Cancer Drug Targets. 2018;18(9):857-868. doi: 10.2174/1568009618666180102102311. Curr Cancer Drug Targets. 2018. PMID: 29295696 Review.
-
SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands.Biomol Eng. 2007 Oct;24(4):381-403. doi: 10.1016/j.bioeng.2007.06.001. Epub 2007 Jun 16. Biomol Eng. 2007. PMID: 17627883 Review.
Cited by
-
Advances in Oligonucleotide Aptamers for NSCLC Targeting.Int J Mol Sci. 2020 Aug 23;21(17):6075. doi: 10.3390/ijms21176075. Int J Mol Sci. 2020. PMID: 32842557 Free PMC article. Review.
-
New Biomarkers in Cancers.Cancers (Basel). 2021 Feb 9;13(4):708. doi: 10.3390/cancers13040708. Cancers (Basel). 2021. PMID: 33572354 Free PMC article.
-
Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies.Front Immunol. 2020 Aug 7;11:1280. doi: 10.3389/fimmu.2020.01280. eCollection 2020. Front Immunol. 2020. PMID: 32849491 Free PMC article. Review.
-
Application of Microfluidics in Detection of Circulating Tumor Cells.Front Bioeng Biotechnol. 2022 May 12;10:907232. doi: 10.3389/fbioe.2022.907232. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35646880 Free PMC article. Review.
-
The Potential of Aptamer-Mediated Liquid Biopsy for Early Detection of Cancer.Int J Mol Sci. 2021 May 25;22(11):5601. doi: 10.3390/ijms22115601. Int J Mol Sci. 2021. PMID: 34070509 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

