Rapid Disappearance of Poliovirus Type 2 (PV2) Immunity in Young Children Following Withdrawal of Oral PV2-Containing Vaccine in Vietnam
- PMID: 30869149
- PMCID: PMC9069936
- DOI: 10.1093/infdis/jiz124
Rapid Disappearance of Poliovirus Type 2 (PV2) Immunity in Young Children Following Withdrawal of Oral PV2-Containing Vaccine in Vietnam
Abstract
Background: Due to global shortage of inactivated poliovirus vaccine and withdrawal of oral vaccine containing poliovirus type 2 (PV2), a PV2-containing vaccine was not used in Vietnam May 2016 to October 2018. We assessed the population immunity gap to PV2.
Methods: A cross-sectional survey in children aged 1-18 months was carried out in January 2018. One blood sample per child was analyzed for presence of poliovirus neutralizing antibodies. In children with detectable anti-PV2 antibodies, a second sample was analyzed 4 months later to distinguish between passive (maternally derived) and active (induced by secondary transmission or vaccination) immunity.
Results: Sera were obtained from 1106/1110 children. Seroprevalence of PV2 antibodies was 87/368 (23.6%) at age 1-7 months, 27/471 (5.7%) at 8-15 months, and 19/267 (7.1%) at 16-18 months. Seroprevalence declined with age in the 1-7 months group; in the 8-18 months group there was no significant change with age. Four months later, 11/87 (14%), 9/27 (32%), and 12/19 (37%) remained seropositive in 1-7, 8-15, and 16-18 months age groups, respectively.
Conclusions: We found declining immunity to PV2, suggesting Vietnam is at risk for an outbreak of type 2 vaccine-derived poliovirus following virus importation or new emergence.
Keywords: Vietnam; eradication; poliomyelitis; vaccination.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Figures
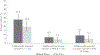
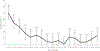
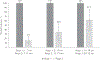
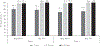
Similar articles
-
Evaluation of vaccine derived poliovirus type 2 outbreak response options: A randomized controlled trial, Karachi, Pakistan.Vaccine. 2018 Mar 20;36(13):1766-1771. doi: 10.1016/j.vaccine.2018.02.051. Epub 2018 Feb 21. Vaccine. 2018. PMID: 29477307 Free PMC article.
-
Poliovirus neutralizing antibody levels among individuals in three regions of Ghana.Ghana Med J. 2019 Jun;53(2):170-180. doi: 10.4314/gmj.v53i2.13. Ghana Med J. 2019. PMID: 31481814 Free PMC article.
-
Does Simultaneous Administration of Bivalent (Types 1 and 3) Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine Induce Mucosal Cross-immunity to Poliovirus Type 2?Clin Infect Dis. 2018 Oct 30;67(suppl_1):S51-S56. doi: 10.1093/cid/ciy604. Clin Infect Dis. 2018. PMID: 30376088 Free PMC article. Clinical Trial.
-
[Possible method of eradication of poliomyelitis as an infection].Zh Mikrobiol Epidemiol Immunobiol. 2012 May-Jun;(3):107-13. Zh Mikrobiol Epidemiol Immunobiol. 2012. PMID: 22830285 Review. Russian.
-
The outbreak of poliomyelitis in Finland in 1984-1985: significance of antigenic variation of type 3 polioviruses and site specificity of antibody responses in antipolio immunizations.Adv Virus Res. 1989;37:243-75. doi: 10.1016/s0065-3527(08)60837-4. Adv Virus Res. 1989. PMID: 2557759 Review. No abstract available.
Cited by
-
Inactivated Poliovirus Vaccine Closing the Type 2 Immunity Gap in Vietnam.J Pediatric Infect Dis Soc. 2022 Sep 29;11(9):413-416. doi: 10.1093/jpids/piac046. J Pediatric Infect Dis Soc. 2022. PMID: 35801634 Free PMC article.
-
One-Year Decline of Poliovirus Antibodies Following Fractional-Dose Inactivated Poliovirus Vaccine.J Infect Dis. 2021 Apr 8;223(7):1214-1221. doi: 10.1093/infdis/jiaa504. J Infect Dis. 2021. PMID: 32798224 Free PMC article. Clinical Trial.
-
Plans for Nationwide Serosurveillance Network in Vietnam.Emerg Infect Dis. 2020 Jan;26(1):e190641. doi: 10.3201/eid2601.190641. Emerg Infect Dis. 2020. PMID: 31855527 Free PMC article.
-
Indian Academy of Pediatrics (IAP) Advisory Committee on Vaccines and Immunization Practices (ACVIP): Recommended Immunization Schedule (2020-21) and Update on Immunization for Children Aged 0 Through 18 Years.Indian Pediatr. 2021 Jan 15;58(1):44-53. doi: 10.1007/s13312-021-2096-7. Epub 2021 Nov 29. Indian Pediatr. 2021. PMID: 33257602 Free PMC article.
References
-
- World Health Organization. Wild poliovirus list. http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli.... Accessed 27 August 2018.
-
- Sutter RW, Platt L, Mach O, Jafari H, Aylward RB. The new polio eradication end game: rationale and supporting evidence. J Infect Dis 2014; 210(Suppl 1):S434–8. - PubMed
-
- World Health Organization. Polio eradication and endgame strategic plan 2013–2018. http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed 18 March 2019.
-
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:934–8. - PubMed
-
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, November 2013 - conclusions and recommendations. Wkly Epidemiol Rec 2014; 89:1–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

