Anatomy and Physiology of Metabotropic Glutamate Receptors in Mammalian and Avian Auditory System
- PMID: 30854519
- PMCID: PMC6405216
- DOI: 10.24966/TAP-7752/100001
Anatomy and Physiology of Metabotropic Glutamate Receptors in Mammalian and Avian Auditory System
Abstract
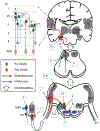
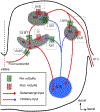
Glutamate, as the major excitatory neurotransmitter used in the vertebrate brain, activates ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs), which mediate fast and slow neuronal actions, respectively. mGluRs play important modulatory roles in many brain areas, forming potential targets for drugs developed to treat brain disorders. Here, we review studies on mGluRs in the mammalian and avian auditory system. Although anatomical expression of mGluRs in the cochlear nucleus has been well characterized, data for other auditory nuclei await more systematic investigations especially at the electron microscopy level. The physiology of mGluRs has been extensively studied using in vitro brain slice preparations, with a focus on the auditory circuitry in the brainstem. These in vitro physiological studies have demonstrated that mGluRs participate in synaptic transmission, regulate ionic homeostasis, induce synaptic plasticity, and maintain the balance between Excitation and Inhibition (E/I) in a variety of auditory structures. However, the modulatory roles of mGluRs in auditory processing remain largely unclear at the system and behavioral levels, and the functions of mGluRs in auditory disorders remain entirely unknown.
Keywords: Auditory processing; Excitotoxicity; Neuromodulation; Neurotransmission; Synaptic plasticity; mGluR.
Conflict of interest statement
Conflict of Interest The author declares no competing financial interests.
Figures
Similar articles
-
Metabotropic glutamate receptors in auditory processing.Neuroscience. 2014 Aug 22;274:429-45. doi: 10.1016/j.neuroscience.2014.05.057. Epub 2014 Jun 5. Neuroscience. 2014. PMID: 24909898 Free PMC article. Review.
-
Activity-dependent synaptic integration and modulation of bilateral excitatory inputs in an auditory coincidence detection circuit.J Physiol. 2018 May 15;596(10):1981-1997. doi: 10.1113/JP275735. Epub 2018 Apr 16. J Physiol. 2018. PMID: 29572827 Free PMC article.
-
Metabotropic glutamate receptors: electrical and chemical signaling properties.Neuroscientist. 2002 Dec;8(6):551-61. doi: 10.1177/1073858402238514. Neuroscientist. 2002. PMID: 12467377 Review.
-
Neurotransmitter- and Release-Mode-Specific Modulation of Inhibitory Transmission by Group I Metabotropic Glutamate Receptors in Central Auditory Neurons of the Mouse.J Neurosci. 2018 Sep 19;38(38):8187-8199. doi: 10.1523/JNEUROSCI.0603-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093538 Free PMC article.
-
Metabotropic glutamate receptors as drug targets.Curr Drug Targets. 2007 May;8(5):651-81. doi: 10.2174/138945007780618544. Curr Drug Targets. 2007. PMID: 17504108 Review.
Cited by
-
Neuromodulation by mGluRs in Sound Localization Circuits in the Auditory Brainstem.Front Neural Circuits. 2020 Nov 5;14:599600. doi: 10.3389/fncir.2020.599600. eCollection 2020. Front Neural Circuits. 2020. PMID: 33224028 Free PMC article. Review.
-
Revisiting the Chicken Auditory Brainstem Response: Frequency Specificity, Threshold Sensitivity, and Cross Species Comparison.Neurosci Insights. 2024 Jan 30;19:26331055241228308. doi: 10.1177/26331055241228308. eCollection 2024. Neurosci Insights. 2024. PMID: 38304551 Free PMC article.
-
Group II Metabotropic Glutamate Receptors Modulate Sound Evoked and Spontaneous Activity in the Mouse Inferior Colliculus.eNeuro. 2021 Jan 15;8(1):ENEURO.0328-20.2020. doi: 10.1523/ENEURO.0328-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33334826 Free PMC article.
-
Focusing on the Emerging Role of Kainate Receptors in the Dorsal Cochlear Nucleus (DCN) and Cerebellum.Int J Mol Sci. 2023 Jan 15;24(2):1718. doi: 10.3390/ijms24021718. Int J Mol Sci. 2023. PMID: 36675230 Free PMC article. Review.
-
Metabotropic Glutamate Receptors in Alzheimer's Disease Synaptic Dysfunction: Therapeutic Opportunities and Hope for the Future.J Alzheimers Dis. 2020;78(4):1345-1361. doi: 10.3233/JAD-201146. J Alzheimers Dis. 2020. PMID: 33325389 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
