Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy
- PMID: 30853401
- PMCID: PMC6642318
- DOI: 10.1016/j.molcel.2019.02.010
Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy
Abstract
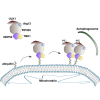
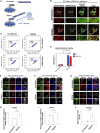
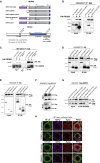
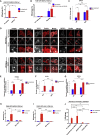
Selective autophagy recycles damaged organelles and clears intracellular pathogens to prevent their aberrant accumulation. How ULK1 kinase is targeted and activated during selective autophagic events remains to be elucidated. In this study, we used chemically inducible dimerization (CID) assays in tandem with CRISPR KO lines to systematically analyze the molecular basis of selective autophagosome biogenesis. We demonstrate that ectopic placement of NDP52 on mitochondria or peroxisomes is sufficient to initiate selective autophagy by focally localizing and activating the ULK1 complex. The capability of NDP52 to induce mitophagy is dependent on its interaction with the FIP200/ULK1 complex, which is facilitated by TBK1. Ectopically tethering ULK1 to cargo bypasses the requirement for autophagy receptors and TBK1. Focal activation of ULK1 occurs independently of AMPK and mTOR. Our findings provide a parsimonious model of selective autophagy, which highlights the coordination of ULK1 complex localization by autophagy receptors and TBK1 as principal drivers of targeted autophagosome biogenesis.
Keywords: ATG13; FIP200; P62; PINK1; Parkin; TAX1BP1; lysosome; mitochondria; mitophagy; optineurin.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The Cargo Receptor NDP52 Initiates Selective Autophagy by Recruiting the ULK Complex to Cytosol-Invading Bacteria.Mol Cell. 2019 Apr 18;74(2):320-329.e6. doi: 10.1016/j.molcel.2019.01.041. Epub 2019 Mar 7. Mol Cell. 2019. PMID: 30853402 Free PMC article.
-
ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy.J Biol Chem. 2009 May 1;284(18):12297-305. doi: 10.1074/jbc.M900573200. Epub 2009 Mar 3. J Biol Chem. 2009. PMID: 19258318 Free PMC article.
-
FIP200 controls the TBK1 activation threshold at SQSTM1/p62-positive condensates.Sci Rep. 2021 Jul 5;11(1):13863. doi: 10.1038/s41598-021-92408-4. Sci Rep. 2021. PMID: 34226595 Free PMC article.
-
[Research progress on the role of TANK-binding kinase 1 in PINK1/Parkin-dependent and -independent mitophagy].Sheng Li Xue Bao. 2024 Feb 25;76(1):161-172. Sheng Li Xue Bao. 2024. PMID: 38444141 Review. Chinese.
-
The mammalian ULK1 complex and autophagy initiation.Essays Biochem. 2017 Dec 12;61(6):585-596. doi: 10.1042/EBC20170021. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233870 Free PMC article. Review.
Cited by
-
PINK1/Parkin Influences Cell Cycle by Sequestering TBK1 at Damaged Mitochondria, Inhibiting Mitosis.Cell Rep. 2019 Oct 1;29(1):225-235.e5. doi: 10.1016/j.celrep.2019.08.085. Cell Rep. 2019. PMID: 31577952 Free PMC article.
-
Mechanisms underlying ubiquitin-driven selective mitochondrial and bacterial autophagy.Mol Cell. 2022 Apr 21;82(8):1501-1513. doi: 10.1016/j.molcel.2022.03.012. Epub 2022 Mar 31. Mol Cell. 2022. PMID: 35364016 Free PMC article. Review.
-
Regulation of Mitophagy by Sirtuin Family Proteins: A Vital Role in Aging and Age-Related Diseases.Front Aging Neurosci. 2022 May 9;14:845330. doi: 10.3389/fnagi.2022.845330. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35615591 Free PMC article. Review.
-
Mitophagy in Diabetic Kidney Disease.Front Cell Dev Biol. 2021 Dec 10;9:778011. doi: 10.3389/fcell.2021.778011. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957109 Free PMC article. Review.
-
Mammalian hybrid pre-autophagosomal structure HyPAS generates autophagosomes.Cell. 2021 Nov 24;184(24):5950-5969.e22. doi: 10.1016/j.cell.2021.10.017. Epub 2021 Nov 5. Cell. 2021. PMID: 34741801 Free PMC article.
References
-
- Alemu E.A., Lamark T., Torgersen K.M., Birgisdottir A.B., Larsen K.B., Jain A., Olsvik H., Øvervatn A., Kirkin V., Johansen T. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 2012;287:39275–39290. - PMC - PubMed
-
- Bach M., Larance M., James D.E., Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem. J. 2011;440:283–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

