Androgen receptor antagonism accelerates disease onset in the SOD1G93A mouse model of amyotrophic lateral sclerosis
- PMID: 30849180
- PMCID: PMC6555856
- DOI: 10.1111/bph.14657
Androgen receptor antagonism accelerates disease onset in the SOD1G93A mouse model of amyotrophic lateral sclerosis
Abstract
Background and purpose: Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease typically more common in males, implicating androgens in progression of both patients and mouse models. Androgen effects are mediated by androgen receptor which is highly expressed in spinal motor neurons and skeletal muscles. To clarify the role of androgen receptors in ALS, we therefore examined the effect of androgen receptor antagonism in the SOD1G93A mouse model.
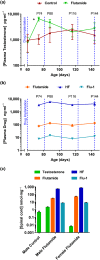
Experimental approach: The androgen receptor antagonist, flutamide, was administered to presymptomatic SOD1G93A mice as a slow-release subcutaneous implant (5 mg·day-1 ). Testosterone, flutamide, and metabolite levels were measured in blood and spinal cord tissue by LC-MS-MS. Effects on disease onset and progression were assessed using motor function tests, survival, muscle, and neuropathological analyses.
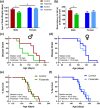
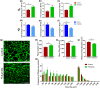
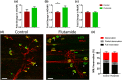
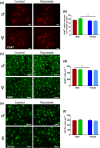
Key results: Flutamide was metabolised to 2-hydroxyflutamide achieving steady-state plasma levels across the study duration and reached the spinal cord at pharmacologically active concentrations. Flutamide treatment accelerated disease onset and locomotor dysfunction in male SOD1G93A mice, but not female mice, without affecting survival. Analysis of hindlimb muscles revealed exacerbation of myofibre atrophy in male SOD1G93A mice treated with flutamide, although motor neuron pathology was not affected.
Conclusion and implications: The androgen receptor antagonist accelerated disease onset in male SOD1G93A mice, leading to exacerbated muscle pathology, consistent with a role of androgens in modulating disease severity, sexual dimorphism, and peripheral pathology in ALS. These results also demonstrate a key contribution of skeletal muscle pathology to disease onset, but not outcome, in this mouse model of ALS.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Dissociation of disease onset, progression and sex differences from androgen receptor levels in a mouse model of amyotrophic lateral sclerosis.Sci Rep. 2021 Apr 29;11(1):9255. doi: 10.1038/s41598-021-88415-0. Sci Rep. 2021. PMID: 33927243 Free PMC article.
-
Androgens affect muscle, motor neuron, and survival in a mouse model of SOD1-related amyotrophic lateral sclerosis.Neurobiol Aging. 2014 Aug;35(8):1929-38. doi: 10.1016/j.neurobiolaging.2014.02.004. Epub 2014 Feb 6. Neurobiol Aging. 2014. PMID: 24630363
-
AAV-NRIP gene therapy ameliorates motor neuron degeneration and muscle atrophy in ALS model mice.Skelet Muscle. 2024 Jul 24;14(1):17. doi: 10.1186/s13395-024-00349-z. Skelet Muscle. 2024. PMID: 39044305 Free PMC article.
-
The Role of Skeletal Muscle in Amyotrophic Lateral Sclerosis.Brain Pathol. 2016 Mar;26(2):227-36. doi: 10.1111/bpa.12350. Brain Pathol. 2016. PMID: 26780251 Free PMC article. Review.
-
Expression Changes of miRNAs in Humans and Animal Models of Amyotrophic Lateral Sclerosis and Their Potential Application for Clinical Diagnosis.Life (Basel). 2024 Sep 6;14(9):1125. doi: 10.3390/life14091125. Life (Basel). 2024. PMID: 39337908 Free PMC article. Review.
Cited by
-
Dissociation of disease onset, progression and sex differences from androgen receptor levels in a mouse model of amyotrophic lateral sclerosis.Sci Rep. 2021 Apr 29;11(1):9255. doi: 10.1038/s41598-021-88415-0. Sci Rep. 2021. PMID: 33927243 Free PMC article.
-
Brain-body mechanisms contribute to sexual dimorphism in amyotrophic lateral sclerosis.Nat Rev Neurol. 2024 Aug;20(8):475-494. doi: 10.1038/s41582-024-00991-7. Epub 2024 Jul 4. Nat Rev Neurol. 2024. PMID: 38965379 Review.
-
The Clinical and Polynucleotide Repeat Expansion Analysis of ATXN2, NOP56, AR and C9orf72 in Patients With ALS From Mainland China.Front Neurol. 2022 May 6;13:811202. doi: 10.3389/fneur.2022.811202. eCollection 2022. Front Neurol. 2022. PMID: 35599735 Free PMC article.
-
Is Dutasteride a Therapeutic Alternative for Amyotrophic Lateral Sclerosis?Biomedicines. 2022 Aug 25;10(9):2084. doi: 10.3390/biomedicines10092084. Biomedicines. 2022. PMID: 36140184 Free PMC article. Review.
-
Androgen-targeting therapeutics mitigate the adverse effect of GnRH agonist on the risk of neurodegenerative disease in men treated for prostate cancer.Cancer Med. 2022 Jul;11(13):2687-2698. doi: 10.1002/cam4.4650. Epub 2022 Mar 16. Cancer Med. 2022. PMID: 35293700 Free PMC article.
References
-
- Aggarwal, T. , Polanco, M. J. , Scaramuzzino, C. , Rocchi, A. , Milioto, C. , Emionite, L. , … Pennuto, M. (2014). Androgens affect muscle, motor neuron, and survival in a mouse model of SOD1‐related amyotrophic lateral sclerosis. Neurobiology of Aging, 35, 1929–1938. 10.1016/j.neurobiolaging.2014.02.004 - DOI - PubMed
-
- Aizawa, Y. , Ikemoto, I. , Kishimoto, K. , Wada, T. , Yamazaki, H. , Ohishi, Y. , … Ueda, M. (2003). Flutamide‐induced hepatic dysfunction in relation to steady‐state plasma concentrations of flutamide and its metabolites. Molecular and Cellular Biochemistry, 252, 149–156. 10.1023/A:1025560513308 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

