Anastasis: recovery from the brink of cell death
- PMID: 30839720
- PMCID: PMC6170572
- DOI: 10.1098/rsos.180442
Anastasis: recovery from the brink of cell death
Erratum in
-
Correction to: 'Anastasis: recovery from the brink of cell death'.R Soc Open Sci. 2018 Oct 10;5(10):181629. doi: 10.1098/rsos.181629. eCollection 2018 Oct. R Soc Open Sci. 2018. PMID: 30475350 Free PMC article.
Abstract
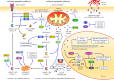
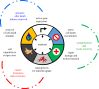
Anastasis is a natural cell recovery phenomenon that rescues cells from the brink of death. Programmed cell death such as apoptosis has been traditionally assumed to be an intrinsically irreversible cascade that commits cells to a rapid and massive demolition. Interestingly, recent studies have demonstrated recovery of dying cells even at the late stages generally considered immutable. Here, we examine the evidence for anastasis in cultured cells and in animals, review findings illuminating the potential mechanisms of action, discuss the challenges of studying anastasis and explore new strategies to uncover the function and regulation of anastasis, the identification of which has wide-ranging physiological, pathological and therapeutic implications.
Keywords: anastasis; apoptosis; mutagenesis; programmed cell death; reversal of apoptosis; reversal of cell death process.
Conflict of interest statement
We have no competing interests.
Figures

Similar articles
-
Molecular signature of anastasis for reversal of apoptosis.F1000Res. 2017 Jan 13;6:43. doi: 10.12688/f1000research.10568.2. eCollection 2017. F1000Res. 2017. PMID: 28299189 Free PMC article.
-
Transcriptomic study of anastasis for reversal of ethanol-induced apoptosis in mouse primary liver cells.Sci Data. 2022 Jul 18;9(1):418. doi: 10.1038/s41597-022-01470-8. Sci Data. 2022. PMID: 35851273 Free PMC article.
-
Detecting Anastasis In Vivo by CaspaseTracker Biosensor.J Vis Exp. 2018 Feb 1;(132):54107. doi: 10.3791/54107. J Vis Exp. 2018. PMID: 29443051 Free PMC article.
-
Anastasis: cell recovery mechanisms and potential role in cancer.Cell Commun Signal. 2022 Jun 3;20(1):81. doi: 10.1186/s12964-022-00880-w. Cell Commun Signal. 2022. PMID: 35659306 Free PMC article. Review.
-
Integration of EMT and cellular survival instincts in reprogramming of programmed cell death to anastasis.Cancer Metastasis Rev. 2020 Jun;39(2):553-566. doi: 10.1007/s10555-020-09866-x. Cancer Metastasis Rev. 2020. PMID: 32020420 Review.
Cited by
-
Anastasis confers ovarian cancer cells increased malignancy through elevated p38 MAPK activation.Cell Death Differ. 2023 Mar;30(3):809-824. doi: 10.1038/s41418-022-01081-1. Epub 2022 Nov 29. Cell Death Differ. 2023. PMID: 36447048 Free PMC article.
-
What Are the Reasons for Continuing Failures in Cancer Therapy? Are Misleading/Inappropriate Preclinical Assays to Be Blamed? Might Some Modern Therapies Cause More Harm than Benefit?Int J Mol Sci. 2022 Oct 30;23(21):13217. doi: 10.3390/ijms232113217. Int J Mol Sci. 2022. PMID: 36362004 Free PMC article. Review.
-
Puncta intended: connecting the dots between autophagy and cell stress networks.Autophagy. 2021 Apr;17(4):1028-1033. doi: 10.1080/15548627.2020.1775394. Epub 2020 Jun 7. Autophagy. 2021. PMID: 32507070 Free PMC article.
-
Probing cell membrane integrity using a histone-targeting protein nanocage displaying precisely positioned fluorophores.Nano Res. 2023;16(1):894-904. doi: 10.1007/s12274-022-4785-5. Epub 2022 Sep 2. Nano Res. 2023. PMID: 36090614 Free PMC article.
-
Exploring the relationship between anastasis and mitochondrial ROS-mediated ferroptosis in metastatic chemoresistant cancers: a call for investigation.Front Immunol. 2024 Jul 2;15:1428920. doi: 10.3389/fimmu.2024.1428920. eCollection 2024. Front Immunol. 2024. PMID: 39015566 Free PMC article. Review.
References
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

