In Search of the Ideal Valve: Optimizing Genetic Modifications to Prevent Bioprosthetic Degeneration
- PMID: 30836101
- PMCID: PMC6656597
- DOI: 10.1016/j.athoracsur.2019.01.054
In Search of the Ideal Valve: Optimizing Genetic Modifications to Prevent Bioprosthetic Degeneration
Abstract
Background: Bioprosthetic heart valves undergo structural degeneration and calcification. Similarities exist in the histopathologic features of explanted bioprosthetic valves and rejected pig tissues and organs after xenotransplantation into nonhuman primates. The development of more durable bioprosthetic valves, namely from genetically modified pigs, could negate the need for the insertion of mechanical prostheses in children and young adults with the requirement for life-long anticoagulation and might avoid the need for reoperation in elderly patients.
Methods: We reviewed the literature (MedlinePlus, PubMed, Google Scholar) through September 1, 2018, under four key terms: (1) bioprosthetic heart valves, (2) xenograft antigens, (3) immunologic responses to bioprosthetic valves, and (4) genetic modification of xenografts.
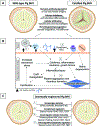
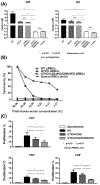
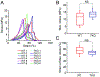
Results: Advances in tissue and organ xenotransplantation have elucidated important immunologic barriers that provide innovative approaches to prevent structural degeneration of bioprosthetic heart valves. The current evidence suggests that bioprosthetic valves derived from genetically modified pigs lacking xenogeneic antigens (namely Gal, Neu5Gc, and Sda), termed triple-knockout pigs, would function considerably longer than current wild-type (genetically unmodified) porcine valves in human recipients.
Conclusions: Preclinical and clinical studies to determine the safety and efficacy of triple-knockout porcine bioprosthetic valves will likely establish that they are more resistant to human immune responses and thus less susceptible to structural degeneration.
Copyright © 2019 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures


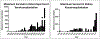
Comment in
-
Developing an Ideal Cardiac Valve Bioprosthesis: Xeno's Paradox.Ann Thorac Surg. 2019 Aug;108(2):319-320. doi: 10.1016/j.athoracsur.2019.02.024. Epub 2019 Mar 18. Ann Thorac Surg. 2019. PMID: 30898562 No abstract available.
Similar articles
-
Porcine bioprosthetic heart valves: The next generation.Am Heart J. 2012 Aug;164(2):177-85. doi: 10.1016/j.ahj.2012.05.011. Am Heart J. 2012. PMID: 22877802 Review.
-
Bioprosthetic heart valves of the future.Xenotransplantation. 2014 Jan-Feb;21(1):1-10. doi: 10.1111/xen.12080. Epub 2014 Jan 21. Xenotransplantation. 2014. PMID: 24444036 Free PMC article. Review.
-
Physical equivalency of wild type and galactose α 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves.Acta Biomater. 2016 Sep 1;41:204-209. doi: 10.1016/j.actbio.2016.06.007. Epub 2016 Jun 4. Acta Biomater. 2016. PMID: 27268480 Free PMC article.
-
Degenerative processes in bioprosthetic mitral valves in juvenile pigs.J Cardiothorac Surg. 2011 May 15;6:72. doi: 10.1186/1749-8090-6-72. J Cardiothorac Surg. 2011. PMID: 21569636 Free PMC article.
-
Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens, GGTA1/β4GalNT2/CMAH.Acta Biomater. 2018 May;72:196-205. doi: 10.1016/j.actbio.2018.03.055. Epub 2018 Apr 7. Acta Biomater. 2018. PMID: 29631050
Cited by
-
Biological Equivalence of GGTA-1 Glycosyltransferase Knockout and Standard Porcine Pericardial Tissue Using 90-Day Mitral Valve Implantation in Adolescent Sheep.Cardiovasc Eng Technol. 2022 Jun;13(3):363-372. doi: 10.1007/s13239-021-00585-0. Epub 2021 Nov 24. Cardiovasc Eng Technol. 2022. PMID: 34820778 Free PMC article.
-
Immune disguise: the mechanisms of Neu5Gc inducing autoimmune and transplant rejection.Genes Immun. 2022 Sep;23(6):175-182. doi: 10.1038/s41435-022-00182-8. Epub 2022 Sep 23. Genes Immun. 2022. PMID: 36151402 Review.
-
In Situ "Humanization" of Porcine Bioprostheses: Demonstration of Tendon Bioprostheses Conversion into Human ACL and Possible Implications for Heart Valve Bioprostheses.Bioengineering (Basel). 2021 Jan 12;8(1):10. doi: 10.3390/bioengineering8010010. Bioengineering (Basel). 2021. PMID: 33445522 Free PMC article. Review.
-
Can Heart Valve Decellularization Be Standardized? A Review of the Parameters Used for the Quality Control of Decellularization Processes.Front Bioeng Biotechnol. 2022 Feb 17;10:830899. doi: 10.3389/fbioe.2022.830899. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35252139 Free PMC article. Review.
-
N-Glycolylneuraminic Acid (Neu5Gc) Null Large Animals by Targeting the CMP-Neu5Gc Hydroxylase (CMAH).Front Immunol. 2019 Oct 15;10:2396. doi: 10.3389/fimmu.2019.02396. eCollection 2019. Front Immunol. 2019. PMID: 31681287 Free PMC article. Review.
References
-
- D'agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:15–23. - PubMed
-
- Head S, Mevlut C, Kappetein P. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017;38:2183–2191. - PubMed
-
- Jamieson W, Munro A, Miyagishima R, Allen P, Burr LH,Tyers GF. Carpentier-Edwards standard porcine bioprosthesis: clinical performance to seventeen years. Ann Thorac Surg 1995;60:999–1006. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

