Prospective application of stem cells to prevent post-operative skeletal fibrosis
- PMID: 30835890
- PMCID: PMC9202416
- DOI: 10.1002/jor.24266
Prospective application of stem cells to prevent post-operative skeletal fibrosis
Abstract
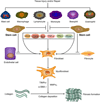
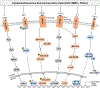
Post-operative skeletal fibrosis is considered one of the major complications causing dysfunction of the skeletal system and compromising the outcomes of clinical treatment. Limited success has been achieved using current therapies; more effective therapies to reduce post-operative skeletal fibrosis are needed. Stem cells possess the ability to repair and regenerate damaged tissue. Numerous studies show that stem cells serve as a promising therapeutic approach for fibrotic diseases in tissues other than the skeletal system by inhibiting the inflammatory response and secreting favorable cytokines through activating specific signaling pathways, acting as so-called medicinal signaling cells. In this review, current therapies are summarized for post-operative skeletal fibrosis. Given that stem cells are used as a promising therapeutic approach for fibrotic diseases, little effort has been undertaken to use stem cells to prevent post-operative skeletal fibrosis. This review aims at providing useful information for the potential application of stem cells in preventing post-operative skeletal fibrosis in the near future. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 37:1236-1245, 2019.
Keywords: epidural fibrosis; intraarticular fibrosis; peritendinous fibrosis; post-operative skeletal fibrosis; stem cell.
© 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures
Similar articles
-
Biomaterial and stem cell-based strategies for skeletal muscle regeneration.J Orthop Res. 2019 Jun;37(6):1246-1262. doi: 10.1002/jor.24212. Epub 2019 Feb 14. J Orthop Res. 2019. PMID: 30604468 Review.
-
Stem cells, angiogenesis and muscle healing: a potential role in massage therapies?Br J Sports Med. 2013 Jun;47(9):556-60. doi: 10.1136/bjsports-2012-091685. Epub 2012 Nov 29. Br J Sports Med. 2013. PMID: 23197410 Review.
-
Pirfenidone reduces subchondral bone loss and fibrosis after murine knee cartilage injury.J Orthop Res. 2018 Jan;36(1):365-376. doi: 10.1002/jor.23635. Epub 2017 Jul 21. J Orthop Res. 2018. PMID: 28646530 Free PMC article.
-
Autophagy Promotes the Survival of Adipose Mesenchymal Stem/Stromal Cells and Enhances Their Therapeutic Effects in Cisplatin-Induced Liver Injury via Modulating TGF-β1/Smad and PI3K/AKT Signaling Pathways.Cells. 2021 Sep 18;10(9):2475. doi: 10.3390/cells10092475. Cells. 2021. PMID: 34572126 Free PMC article.
-
Immunomodulatory capacity of the local mesenchymal stem cells transplantation after severe skeletal muscle injury in female rats.Immunopharmacol Immunotoxicol. 2016 Dec;38(6):414-422. doi: 10.1080/08923973.2016.1222617. Epub 2016 Aug 25. Immunopharmacol Immunotoxicol. 2016. PMID: 27560658
Cited by
-
Adipose stem cells exhibit mechanical memory and reduce fibrotic contracture in a rat elbow injury model.FASEB J. 2020 Sep;34(9):12976-12990. doi: 10.1096/fj.202001274R. Epub 2020 Aug 9. FASEB J. 2020. PMID: 33411380 Free PMC article.
-
Tanshinone IIA regulates fibroblast proliferation and migration and post-surgery arthrofibrosis through the autophagy-mediated PI3K and AMPK-mTOR signaling pathway.Am J Transl Res. 2021 Feb 15;13(2):565-584. eCollection 2021. Am J Transl Res. 2021. PMID: 33594310 Free PMC article.
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Fibroblast Proliferation by Regulating MT2A to Reduce Knee Arthrofibrosis.Stem Cells Int. 2023 Apr 12;2023:9067621. doi: 10.1155/2023/9067621. eCollection 2023. Stem Cells Int. 2023. PMID: 37091533 Free PMC article.
References
-
- Rockey DC, Bell PD, Hill JA. 2015. Fibrosis--a common pathway to organ injury and failure. The New England journal of medicine 372:1138–1149. - PubMed
-
- Lemos DR, Babaeijandaghi F, Low M, et al. 2015. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nature medicine 21:786–794. - PubMed
-
- Li X, Yan L, Wang J, et al. 2013. Comparison of the effects of mitomycin C and 10-hydroxycamptothecin on an experimental intraarticular adhesion model in rabbits. European journal of pharmacology 703:42–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

