Multiplex PCR methods for detection of several viruses associated with canine respiratory and enteric diseases
- PMID: 30830947
- PMCID: PMC6398926
- DOI: 10.1371/journal.pone.0213295
Multiplex PCR methods for detection of several viruses associated with canine respiratory and enteric diseases
Abstract
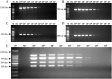
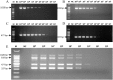
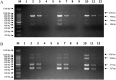
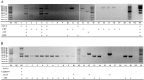
Viral respiratory and intestinal infections are the most common causes of canine viral illness. Infection with multiple pathogens occurs in many cases. Rapid diagnosis of these multiple infections is important for providing timely and effective treatment. To improve diagnosis, in this study, two new multiplex polymerase chain reactions (mPCRs) were developed for simultaneous detection of canine respiratory viruses (CRV) and canine enteric viruses (CEV) using two separate primer mixes. The viruses included canine adenovirus type 2 (CAV-2), canine distemper virus (CDV), canine influenza virus (CIV), canine parainfluenza virus (CPIV), canine circovirus (CanineCV), canine coronavirus (CCoV) and canine parvovirus (CPV). The sensitivity of the mPCR results showed that the detection limit of both mPCR methods was 1×104 viral copies. Twenty nasal swabs (NS) and 20 anal swabs (AS) collected from dogs with symptoms of respiratory disease or enteric disease were evaluated using the novel mPCR methods as a clinical test. The mPCR protocols, when applied to these respiratory specimens and intestinal samples, could detect 7 viruses simultaneously, allowing rapid investigation of CRV (CAV-2, CDV, CIV and CPIV) and CEV (CAV-2, CanineCV, CCoV and CPV) status and prompt evaluation of coinfection. Our study provides an effective and accurate tool for rapid differential diagnosis and epidemiological surveillance in dogs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A multiplex PCR method for the simultaneous detection of three viruses associated with canine viral enteric infections.Arch Virol. 2018 Aug;163(8):2133-2138. doi: 10.1007/s00705-018-3828-4. Epub 2018 Apr 19. Arch Virol. 2018. PMID: 29675651 Free PMC article.
-
Identification of enteric viruses circulating in a dog population with low vaccine coverage.Braz J Microbiol. 2018 Oct-Dec;49(4):790-794. doi: 10.1016/j.bjm.2018.02.006. Epub 2018 Mar 15. Braz J Microbiol. 2018. PMID: 29588198 Free PMC article.
-
Rapid diagnosis of canine respiratory coronavirus, canine influenza virus, canine distemper virus and canine parainfluenza virus with a Taqman probe-based multiplex real-time PCR.J Virol Methods. 2024 Jul;328:114960. doi: 10.1016/j.jviromet.2024.114960. Epub 2024 May 31. J Virol Methods. 2024. PMID: 38823586
-
Genetic evolution of canine coronavirus and recent advances in prophylaxis.Vet Res. 2006 Mar-Apr;37(2):191-200. doi: 10.1051/vetres:2005053. Vet Res. 2006. PMID: 16472519 Review.
-
Aetiology of Canine Infectious Respiratory Disease Complex and Prevalence of its Pathogens in Europe.J Comp Pathol. 2020 Apr;176:86-108. doi: 10.1016/j.jcpa.2020.02.005. Epub 2020 Mar 17. J Comp Pathol. 2020. PMID: 32359641 Free PMC article. Review.
Cited by
-
Preparation for the next pandemic: challenges in strengthening surveillance.Emerg Microbes Infect. 2023 Dec;12(2):2240441. doi: 10.1080/22221751.2023.2240441. Emerg Microbes Infect. 2023. PMID: 37474466 Free PMC article.
-
A systematic review and meta-analysis of canine enteric coronavirus prevalence in dogs of mainland China.Virol J. 2024 Jul 9;21(1):155. doi: 10.1186/s12985-024-02425-8. Virol J. 2024. PMID: 38982509 Free PMC article. Review.
-
Prolonged Infection of Canine Distemper Virus in a Mixed-Breed Dog.Vet Sci. 2021 Apr 11;8(4):61. doi: 10.3390/vetsci8040061. Vet Sci. 2021. PMID: 33920469 Free PMC article.
-
Fluorescent Biosensors for the Detection of Viruses Using Graphene and Two-Dimensional Carbon Nanomaterials.Biosensors (Basel). 2022 Jun 27;12(7):460. doi: 10.3390/bios12070460. Biosensors (Basel). 2022. PMID: 35884263 Free PMC article. Review.
-
Canine Circovirus Suppresses the Type I Interferon Response and Protein Expression but Promotes CPV-2 Replication.Int J Mol Sci. 2022 Jun 7;23(12):6382. doi: 10.3390/ijms23126382. Int J Mol Sci. 2022. PMID: 35742826 Free PMC article.
References
-
- Jeoung HY, Song DS, Jeong WS, Lee WH, Song JY, An DJ. Simultaneous detection of canine respiratory disease associated viruses by a multiplex reverse transcription-polymerase chain reaction assay. The Journal of veterinary medical science. 2013;75(1):103–6. . - PubMed
-
- Posuwan N, Payungporn S, Thontiravong A, Kitikoon P, Amonsin A, Poovorawan Y. Prevalence of respiratory viruses isolated from dogs in Thailand during 2008–2009. Asian Biomedicine. 2010;4(4):563–9.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

