Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation
- PMID: 30827897
- PMCID: PMC6907693
- DOI: 10.1016/j.devcel.2019.01.027
Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation
Abstract
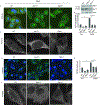
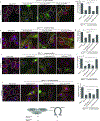
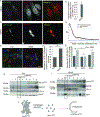
Syntaxin 17 (Stx17) has been implicated in autophagosome-lysosome fusion. Here, we report that Stx17 functions in assembly of protein complexes during autophagy initiation. Stx17 is phosphorylated by TBK1 whereby phospho-Stx17 controls the formation of the ATG13+FIP200+ mammalian pre-autophagosomal structure (mPAS) in response to induction of autophagy. TBK1 phosphorylates Stx17 at S202. During autophagy induction, Stx17pS202 transfers from the Golgi, where its steady-state pools localize, to the ATG13+FIP200+ mPAS. Stx17pS202 was in complexes with ATG13 and FIP200, whereas its non-phosphorylatable mutant Stx17S202A was not. Stx17 or TBK1 knockouts blocked ATG13 and FIP200 puncta formation. Stx17 or TBK1 knockouts reduced the formation of ATG13 protein complexes with FIP200 and ULK1. Endogenous Stx17pS202 colocalized with LC3B following induction of autophagy. Stx17 knockout diminished LC3 response and reduced sequestration of the prototypical bulk autophagy cargo lactate dehydrogenase. We conclude that Stx17 is a TBK1 substrate and that together they orchestrate assembly of mPAS.
Keywords: TBK1; ULK1; autophagy; pre-autophagosomal structure.
Published by Elsevier Inc.
Conflict of interest statement
Figures




Similar articles
-
Spatiotemporal Control of ULK1 Activation by NDP52 and TBK1 during Selective Autophagy.Mol Cell. 2019 Apr 18;74(2):347-362.e6. doi: 10.1016/j.molcel.2019.02.010. Epub 2019 Mar 7. Mol Cell. 2019. PMID: 30853401 Free PMC article.
-
ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy.J Biol Chem. 2009 May 1;284(18):12297-305. doi: 10.1074/jbc.M900573200. Epub 2009 Mar 3. J Biol Chem. 2009. PMID: 19258318 Free PMC article.
-
YKT6 as a second SNARE protein of mammalian autophagosomes.Autophagy. 2019 Jan;15(1):176-177. doi: 10.1080/15548627.2018.1532262. Epub 2018 Oct 13. Autophagy. 2019. PMID: 30290708 Free PMC article.
-
The mammalian ULK1 complex and autophagy initiation.Essays Biochem. 2017 Dec 12;61(6):585-596. doi: 10.1042/EBC20170021. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233870 Free PMC article. Review.
-
Evolutionarily conserved role and physiological relevance of a STX17/Syx17 (syntaxin 17)-containing SNARE complex in autophagosome fusion with endosomes and lysosomes.Autophagy. 2013 Oct;9(10):1642-6. doi: 10.4161/auto.25684. Epub 2013 Jul 22. Autophagy. 2013. PMID: 24113031 Review.
Cited by
-
FIP200 Suppresses Immune Checkpoint Therapy Responses in Breast Cancers by Limiting AZI2/TBK1/IRF Signaling Independent of Its Canonical Autophagy Function.Cancer Res. 2020 Sep 1;80(17):3580-3592. doi: 10.1158/0008-5472.CAN-20-0519. Epub 2020 Jun 24. Cancer Res. 2020. PMID: 32580962 Free PMC article.
-
Unraveling the Intricacies of Autophagy and Mitophagy: Implications in Cancer Biology.Cells. 2023 Nov 30;12(23):2742. doi: 10.3390/cells12232742. Cells. 2023. PMID: 38067169 Free PMC article. Review.
-
Targeting TANK-binding kinase 1 (TBK1) in cancer.Expert Opin Ther Targets. 2020 Nov;24(11):1065-1078. doi: 10.1080/14728222.2020.1826929. Epub 2020 Oct 5. Expert Opin Ther Targets. 2020. PMID: 32962465 Free PMC article. Review.
-
ATG5 provides host protection acting as a switch in the atg8ylation cascade between autophagy and secretion.Dev Cell. 2023 May 22;58(10):866-884.e8. doi: 10.1016/j.devcel.2023.03.014. Epub 2023 Apr 12. Dev Cell. 2023. PMID: 37054706 Free PMC article.
-
Update on Autophagy Inhibitors in Cancer: Opening up to a Therapeutic Combination with Immune Checkpoint Inhibitors.Cells. 2023 Jun 23;12(13):1702. doi: 10.3390/cells12131702. Cells. 2023. PMID: 37443736 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/B/000C0413/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 AI042999/AI/NIAID NIH HHS/United States
- BB/K019155/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- P20 GM121176/GM/NIGMS NIH HHS/United States
- R37 AI042999/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

