Taming the Triskelion: Bacterial Manipulation of Clathrin
- PMID: 30814130
- PMCID: PMC6684001
- DOI: 10.1128/MMBR.00058-18
Taming the Triskelion: Bacterial Manipulation of Clathrin
Abstract
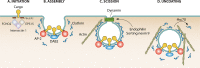
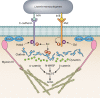
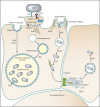
The entry of pathogens into nonphagocytic host cells has received much attention in the past three decades, revealing a vast array of strategies employed by bacteria and viruses. A method of internalization that has been extensively studied in the context of viral infections is the use of the clathrin-mediated pathway. More recently, a role for clathrin in the entry of some intracellular bacterial pathogens was discovered. Classically, clathrin-mediated endocytosis was thought to accommodate internalization only of particles smaller than 150 nm; however, this was challenged upon the discovery that Listeria monocytogenes requires clathrin to enter eukaryotic cells. Now, with discoveries that clathrin is required during other stages of some bacterial infections, another paradigm shift is occurring. There is a more diverse impact of clathrin during infection than previously thought. Much of the recent data describing clathrin utilization in processes such as bacterial attachment, cell-to-cell spread and intracellular growth may be due to newly discovered divergent roles of clathrin in the cell. Not only does clathrin act to facilitate endocytosis from the plasma membrane, but it also participates in budding from endosomes and the Golgi apparatus and in mitosis. Here, the manipulation of clathrin processes by bacterial pathogens, including its traditional role during invasion and alternative ways in which clathrin supports bacterial infection, is discussed. Researching clathrin in the context of bacterial infections will reveal new insights that inform our understanding of host-pathogen interactions and allow researchers to fully appreciate the diverse roles of clathrin in the eukaryotic cell.
Keywords: Coxiella burnetii; EPEC; Listeria monocytogenes; Shigella flexneri; Staphylococcus aureus; adaptor proteins; autophagy; bacterial replication; clathrin; pathogen internalization.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
The role of clathrin-dependent endocytosis in bacterial internalization.Trends Cell Biol. 2006 Oct;16(10):499-504. doi: 10.1016/j.tcb.2006.08.005. Epub 2006 Sep 8. Trends Cell Biol. 2006. PMID: 16962776 Free PMC article. Review.
-
The non-canonical roles of clathrin and actin in pathogen internalization, egress and spread.Nat Rev Microbiol. 2013 Aug;11(8):551-60. doi: 10.1038/nrmicro3072. Nat Rev Microbiol. 2013. PMID: 24020073 Review.
-
Non-classical use of clathrin during bacterial infections.J Microsc. 2008 Sep;231(3):524-8. doi: 10.1111/j.1365-2818.2008.02065.x. J Microsc. 2008. PMID: 18755008 Review.
-
Invasive and adherent bacterial pathogens co-Opt host clathrin for infection.Cell Host Microbe. 2007 Nov 15;2(5):340-51. doi: 10.1016/j.chom.2007.10.001. Cell Host Microbe. 2007. PMID: 18005755 Free PMC article.
-
Bub1 Facilitates Virus Entry through Endocytosis in a Model of Drosophila Pathogenesis.J Virol. 2018 Aug 29;92(18):e00254-18. doi: 10.1128/JVI.00254-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976667 Free PMC article.
Cited by
-
Imaging Endocytosis Dynamics in Health and Disease.Membranes (Basel). 2022 Apr 1;12(4):393. doi: 10.3390/membranes12040393. Membranes (Basel). 2022. PMID: 35448364 Free PMC article. Review.
-
Mastering the control of the Rho transcription factor for biotechnological applications.Appl Microbiol Biotechnol. 2021 May;105(10):4053-4071. doi: 10.1007/s00253-021-11326-7. Epub 2021 May 8. Appl Microbiol Biotechnol. 2021. PMID: 33963893 Review.
-
SARS-CoV-2, periodontal pathogens, and host factors: The trinity of oral post-acute sequelae of COVID-19.Rev Med Virol. 2024 May;34(3):e2543. doi: 10.1002/rmv.2543. Rev Med Virol. 2024. PMID: 38782605 Review.
-
Why Does Piscirickettsia salmonis Break the Immunological Paradigm in Farmed Salmon? Biological Context to Understand the Relative Control of Piscirickettsiosis.Front Immunol. 2022 Mar 21;13:856896. doi: 10.3389/fimmu.2022.856896. eCollection 2022. Front Immunol. 2022. PMID: 35386699 Free PMC article. Review.
-
Endocytosis of abiotic nanomaterials and nanobiovectors: Inhibition of membrane trafficking.Nano Today. 2021 Oct;40:101279. doi: 10.1016/j.nantod.2021.101279. Epub 2021 Sep 8. Nano Today. 2021. PMID: 34518771 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

