Agonist-induced desensitisation of β3 -adrenoceptors: Where, when, and how?
- PMID: 30809805
- PMCID: PMC6592865
- DOI: 10.1111/bph.14633
Agonist-induced desensitisation of β3 -adrenoceptors: Where, when, and how?
Abstract
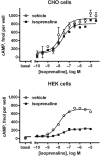
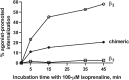
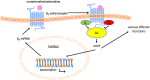
β3 -Adrenoceptor agonists have proven useful in the treatment of overactive bladder syndrome, but it is not known whether their efficacy during chronic administration may be limited by receptor-induced desensitisation. Whereas the β2 -adrenoceptor has phosphorylation sites that are important for desensitisation, the β3 -adrenoceptor lacks these; therefore, it had been assumed that β3 -adrenoceptors are largely resistant to agonist-induced desensitisation. While all direct comparative studies demonstrate that β3 -adrenoceptors are less susceptible to desensitisation than β2 -adrenoceptors, desensitisation of β3 -adrenoceptors has been observed in many models and treatment settings. Chimeric β2 - and β3 -adrenoceptors have demonstrated that the C-terminal tail of the receptor plays an important role in the relative resistance to desensitisation but is not the only relevant factor. While the evidence from some models, such as transfected CHO cells, is inconsistent, it appears that desensitisation is observed more often after long-term (hours to days) than short-term (minutes to hours) agonist exposure. When it occurs, desensitisation of β3 -adrenoceptors can involve multiple levels including down-regulation of its mRNA and the receptor protein and alterations in post-receptor signalling events. The relative contributions of these mechanistic factors apparently depend on the cell type under investigation. Which if any of these factors is applicable to the human urinary bladder remains to be determined. LINKED ARTICLES: This article is part of a themed section on Adrenoceptors-New Roles for Old Players. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.14/issuetoc.
© 2019 The British Pharmacological Society.
Conflict of interest statement
M.C.M. is a consultant to Astellas and a consultant and shareholder of Velicept.
Figures
Similar articles
-
β3 -Adrenoceptors in the normal and diseased urinary bladder-What are the open questions?Br J Pharmacol. 2019 Jul;176(14):2525-2538. doi: 10.1111/bph.14658. Epub 2019 May 3. Br J Pharmacol. 2019. PMID: 30868554 Free PMC article. Review.
-
β-Adrenoceptors as drug targets in melanoma: novel preclinical evidence for a role of β3 -adrenoceptors.Br J Pharmacol. 2019 Jul;176(14):2496-2508. doi: 10.1111/bph.14552. Epub 2018 Dec 18. Br J Pharmacol. 2019. PMID: 30471093 Free PMC article. Review.
-
Cardiac β3 -adrenoceptors-A role in human pathophysiology?Br J Pharmacol. 2019 Jul;176(14):2482-2495. doi: 10.1111/bph.14635. Epub 2019 Apr 22. Br J Pharmacol. 2019. PMID: 30801686 Free PMC article. Review.
-
β3 -Adrenoceptor as a potential immuno-suppressor agent in melanoma.Br J Pharmacol. 2019 Jul;176(14):2509-2524. doi: 10.1111/bph.14660. Epub 2019 May 9. Br J Pharmacol. 2019. PMID: 30874296 Free PMC article.
-
The odd sibling: features of β3-adrenoceptor pharmacology.Mol Pharmacol. 2014 Nov;86(5):479-84. doi: 10.1124/mol.114.092817. Epub 2014 Jun 2. Mol Pharmacol. 2014. PMID: 24890609 Review.
Cited by
-
Agonism of β3-Adrenoceptors Inhibits Pathological Retinal Angiogenesis in the Model of Oxygen-Induced Retinopathy.Invest Ophthalmol Vis Sci. 2024 Aug 1;65(10):34. doi: 10.1167/iovs.65.10.34. Invest Ophthalmol Vis Sci. 2024. PMID: 39186263 Free PMC article.
-
Role of β3-Adrenergic Receptor in Bone Marrow Transplant as Therapeutical Support in Cancer.Front Oncol. 2022 Jun 8;12:889634. doi: 10.3389/fonc.2022.889634. eCollection 2022. Front Oncol. 2022. PMID: 35756654 Free PMC article.
-
β3-adrenergic receptor on tumor-infiltrating lymphocytes sustains IFN-γ-dependent PD-L1 expression and impairs anti-tumor immunity in neuroblastoma.Cancer Gene Ther. 2023 Jun;30(6):890-904. doi: 10.1038/s41417-023-00599-x. Epub 2023 Feb 28. Cancer Gene Ther. 2023. PMID: 36854895 Free PMC article.
-
β3 -Adrenoceptors in the normal and diseased urinary bladder-What are the open questions?Br J Pharmacol. 2019 Jul;176(14):2525-2538. doi: 10.1111/bph.14658. Epub 2019 May 3. Br J Pharmacol. 2019. PMID: 30868554 Free PMC article. Review.
-
Upregulation of β3-adrenoceptors-a general marker of and protective mechanism against hypoxia?Naunyn Schmiedebergs Arch Pharmacol. 2020 Feb;393(2):141-146. doi: 10.1007/s00210-019-01780-6. Epub 2019 Dec 18. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31853614 Review.
References
-
- Amour, J. , Loyer, X. , Le Guen, M. , Mabrouk, N. , David, J.‐S. , Camors, E. , … Riou, B. (2007). Altered contractile response due to increased β3‐adrenoceptor stimulation in diabetic cardiomyopathy. The role of nitric oxide synthase 1‐derived nitric oxide. Anesthesiology, 107, 452–460. 10.1097/01.anes.0000278909.40408.24 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

