CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment
- PMID: 30804938
- PMCID: PMC6370640
- DOI: 10.3389/fimmu.2019.00128
CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment
Abstract
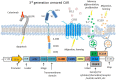
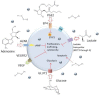
Chimeric antigen receptor (CAR) T cells, T cells that have been genetically engineered to express a receptor that recognizes a specific antigen, have given rise to breakthroughs in treating hematological malignancies. However, their success in treating solid tumors has been limited. The unique challenges posed to CAR T cell therapy by solid tumors can be described in three steps: finding, entering, and surviving in the tumor. The use of dual CAR designs that recognize multiple antigens at once and local administration of CAR T cells are both strategies that have been used to overcome the hurdle of localization to the tumor. Additionally, the immunosuppressive tumor microenvironment has implications for T cell function in terms of differentiation and exhaustion, and combining CARs with checkpoint blockade or depletion of other suppressive factors in the microenvironment has shown very promising results to mitigate the phenomenon of T cell exhaustion. Finally, identifying and overcoming mechanisms associated with dysfunction in CAR T cells is of vital importance to generating CAR T cells that can proliferate and successfully eliminate tumor cells. The structure and costimulatory domains chosen for the CAR may play an important role in the overall function of CAR T cells in the TME, and "armored" CARs that secrete cytokines and third- and fourth-generation CARs with multiple costimulatory domains offer ways to enhance CAR T cell function.
Keywords: T cell; adoptive T cell immunotherapy; chimeric antigen receptor; engineered T cells; solid tumors.
Figures

Similar articles
-
CAR T Cell Therapy for Solid Tumors.Annu Rev Med. 2017 Jan 14;68:139-152. doi: 10.1146/annurev-med-062315-120245. Epub 2016 Nov 17. Annu Rev Med. 2017. PMID: 27860544 Review.
-
Perspectives on Chimeric Antigen Receptor T-Cell Immunotherapy for Solid Tumors.Front Immunol. 2018 May 22;9:1104. doi: 10.3389/fimmu.2018.01104. eCollection 2018. Front Immunol. 2018. PMID: 29872437 Free PMC article. Review.
-
Engineering CAR-T Cells for Next-Generation Cancer Therapy.Cancer Cell. 2020 Oct 12;38(4):473-488. doi: 10.1016/j.ccell.2020.07.005. Epub 2020 Jul 30. Cancer Cell. 2020. PMID: 32735779 Review.
-
Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors.Mol Cancer. 2018 Jan 12;17(1):7. doi: 10.1186/s12943-018-0759-3. Mol Cancer. 2018. PMID: 29329591 Free PMC article. Review.
-
Making CAR T Cells a Solid Option for Solid Tumors.Front Immunol. 2018 Nov 8;9:2593. doi: 10.3389/fimmu.2018.02593. eCollection 2018. Front Immunol. 2018. PMID: 30467505 Free PMC article. Review.
Cited by
-
Mechanisms of Resistance to Immunotherapy in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2023 Nov 3;10:1955-1971. doi: 10.2147/JHC.S291553. eCollection 2023. J Hepatocell Carcinoma. 2023. PMID: 37941812 Free PMC article. Review.
-
CAR-T Cell Therapy-An Overview of Targets in Gastric Cancer.J Clin Med. 2020 Jun 17;9(6):1894. doi: 10.3390/jcm9061894. J Clin Med. 2020. PMID: 32560392 Free PMC article. Review.
-
Harnessing bioengineered myeloid progenitors for precision immunotherapies.NPJ Regen Med. 2023 Dec 12;8(1):66. doi: 10.1038/s41536-023-00343-x. NPJ Regen Med. 2023. PMID: 38086850 Free PMC article. Review.
-
CRISPR/Cas9 in Cancer Immunotherapy: Animal Models and Human Clinical Trials.Genes (Basel). 2020 Aug 11;11(8):921. doi: 10.3390/genes11080921. Genes (Basel). 2020. PMID: 32796761 Free PMC article. Review.
-
Innovative CAR-T Cell Therapy for Solid Tumor; Current Duel between CAR-T Spear and Tumor Shield.Cancers (Basel). 2020 Jul 28;12(8):2087. doi: 10.3390/cancers12082087. Cancers (Basel). 2020. PMID: 32731404 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

