Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment
- PMID: 30802149
- PMCID: PMC6463916
- DOI: 10.1096/fj.201802067RR
Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment
Abstract
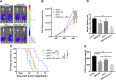
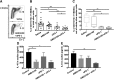
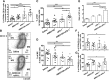
Blockade of immune-checkpoint programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 can enhance effector T-cell responses. However, the lack of response in many patients to checkpoint-inhibitor therapies emphasizes the need for combination immunotherapies to pursue maximal antitumor efficacy. We have previously demonstrated that antagonism of C-X-C chemokine receptor type 4 (CXCR4) by plerixafor (AMD3100) can decrease regulatory T (Treg)-cell intratumoral infiltration. Therefore, a combination of these 2 therapies might increase antitumor effects. Here, we evaluated the antitumor efficacy of AMD3100 and anti-PD-1 (αPD-1) antibody alone or in combination in an immunocompetent syngeneic mouse model of ovarian cancer. We found that AMD3100, a highly specific CXCR4 antagonist, directly down-regulated the expression of both C-X-C motif chemokine 12 (CXCL12) and CXCR4 in vitro and in vivo in tumor cells. AMD3100 and αPD-1 significantly inhibited tumor growth and prolonged the survival of tumor-bearing mice when given as monotherapy. Combination of these 2 agents significantly enhanced antitumor effects compared with single-agent administration. Benefits of tumor control and animal survival were associated with immunomodulation mediated by these 2 agents, which were characterized by increased effector T-cell infiltration, increased effector T-cell function, and increased memory T cells in tumor microenvironment. Intratumoral Treg cells were decreased, and conversion of Treg cells into T helper cells was increased by AMD3100 treatment. Intratumoral myeloid-derived suppressor cells were decreased by the combined treatment, which was associated with decreased IL-10 and IL-6 in the ascites. Also, the combination therapy decreased suppressive leukocytes and facilitated M2-to-M1 macrophage polarization in the tumor. These results suggest that AMD3100 could be used to target the CXCR4-CXCL12 axis to inhibit tumor growth and prevent multifaceted immunosuppression alone or in combination with αPD-1 in ovarian cancer, which could be clinically relevant to patients with this disease.-Zeng, Y., Li, B., Liang, Y., Reeves, P. M., Qu, X., Ran, C., Liu, Q., Callahan, M. V., Sluder, A. E., Gelfand, J. A., Chen, H., Poznansky, M. C. Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment.
Keywords: CXCR4 antagonist; combination immunotherapy; immune checkpoint inhibitor.
Conflict of interest statement
The ID8 cell line was a kind gift from Dr. Kathy Roby (University of Kansas Medical Center, Kansas City, KS, USA). This work was supported by the Vaccine and Immunotherapy Center (VIC) Innovation Fund and the Massachusetts General Hospital (MGH) Research Scholar Award (to H.C., Y.Z., and M.C.P.). Cytometric findings reported here were performed in the MGH Department of Pathology Flow and Image Cytometry Research Core, which is supported by the U.S. National Institutes of Health (NIH) Shared Instrumentation Grants 1S10OD012027-01A1, 1S10OD016372-01, 1S10RR020936-01, and 1S10RR023440-01A1. M.C.P. is the Scientific Founder of AperiSys. The remaining authors declare no conflicts of interest.
Figures




Similar articles
-
Nanoparticle-mediated blockade of CXCL12/CXCR4 signaling enhances glioblastoma immunotherapy: Monitoring early responses with MRI radiomics.Acta Biomater. 2024 Mar 15;177:414-430. doi: 10.1016/j.actbio.2024.02.007. Epub 2024 Feb 14. Acta Biomater. 2024. PMID: 38360292
-
TGF-β1-Induced SOX18 Elevation Promotes Hepatocellular Carcinoma Progression and Metastasis Through Transcriptionally Upregulating PD-L1 and CXCL12.Gastroenterology. 2024 Jul;167(2):264-280. doi: 10.1053/j.gastro.2024.02.025. Epub 2024 Feb 27. Gastroenterology. 2024. PMID: 38417530
-
SDF-1/CXCR4 axis facilitates myeloid-derived suppressor cells accumulation in osteosarcoma microenvironment and blunts the response to anti-PD-1 therapy.Int Immunopharmacol. 2019 Oct;75:105818. doi: 10.1016/j.intimp.2019.105818. Epub 2019 Aug 19. Int Immunopharmacol. 2019. PMID: 31437795
-
Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer.J Gynecol Oncol. 2017 Sep;28(5):e64. doi: 10.3802/jgo.2017.28.e64. Epub 2017 Jun 5. J Gynecol Oncol. 2017. PMID: 28657225 Free PMC article. Review.
-
CXCR4 antagonist AMD3100 (plerixafor): From an impurity to a therapeutic agent.Pharmacol Res. 2020 Sep;159:105010. doi: 10.1016/j.phrs.2020.105010. Epub 2020 Jun 13. Pharmacol Res. 2020. PMID: 32544428 Review.
Cited by
-
Beyond Cell Motility: The Expanding Roles of Chemokines and Their Receptors in Malignancy.Front Immunol. 2020 Jun 4;11:952. doi: 10.3389/fimmu.2020.00952. eCollection 2020. Front Immunol. 2020. PMID: 32582148 Free PMC article. Review.
-
CXCR4 and CXCR7 Signaling Pathways: A Focus on the Cross-Talk Between Cancer Cells and Tumor Microenvironment.Front Oncol. 2021 Apr 15;11:591386. doi: 10.3389/fonc.2021.591386. eCollection 2021. Front Oncol. 2021. PMID: 33937018 Free PMC article. Review.
-
Chemokines as Regulators of Neutrophils: Focus on Tumors, Therapeutic Targeting, and Immunotherapy.Cancers (Basel). 2022 Jan 28;14(3):680. doi: 10.3390/cancers14030680. Cancers (Basel). 2022. PMID: 35158948 Free PMC article. Review.
-
Targeting Myeloid-Derived Suppressor Cells to Enhance the Antitumor Efficacy of Immune Checkpoint Blockade Therapy.Front Immunol. 2021 Dec 22;12:754196. doi: 10.3389/fimmu.2021.754196. eCollection 2021. Front Immunol. 2021. PMID: 35003065 Free PMC article. Review.
-
Impact of the Chemokine Receptors CXCR4 and CXCR7 on Clinical Outcome in Adrenocortical Carcinoma.Front Endocrinol (Lausanne). 2020 Nov 13;11:597878. doi: 10.3389/fendo.2020.597878. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33281749 Free PMC article.
References
-
- Siegel R., DeSantis C., Virgo K., Stein K., Mariotto A., Smith T., Cooper D., Gansler T., Lerro C., Fedewa S., Lin C., Leach C., Cannady R. S., Cho H., Scoppa S., Hachey M., Kirch R., Jemal A., Ward E. (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 62, 220–241 - PubMed
-
- Conrad C., Gregorio J., Wang Y. H., Ito T., Meller S., Hanabuchi S., Anderson S., Atkinson N., Ramirez P. T., Liu Y. J., Freedman R., Gilliet M. (2012) Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 72, 5240–5249 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

