Antidepressant-relevant concentrations of the ketamine metabolite (2 R,6 R)-hydroxynorketamine do not block NMDA receptor function
- PMID: 30796190
- PMCID: PMC6421428
- DOI: 10.1073/pnas.1816071116
Antidepressant-relevant concentrations of the ketamine metabolite (2 R,6 R)-hydroxynorketamine do not block NMDA receptor function
Abstract
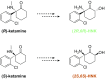
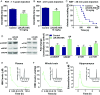
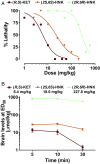
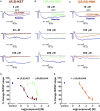
Preclinical studies indicate that (2R,6R)-hydroxynorketamine (HNK) is a putative fast-acting antidepressant candidate. Although inhibition of NMDA-type glutamate receptors (NMDARs) is one mechanism proposed to underlie ketamine's antidepressant and adverse effects, the potency of (2R,6R)-HNK to inhibit NMDARs has not been established. We used a multidisciplinary approach to determine the effects of (2R,6R)-HNK on NMDAR function. Antidepressant-relevant behavioral responses and (2R,6R)-HNK levels in the extracellular compartment of the hippocampus were measured following systemic (2R,6R)-HNK administration in mice. The effects of ketamine, (2R,6R)-HNK, and, in some cases, the (2S,6S)-HNK stereoisomer were evaluated on the following: (i) NMDA-induced lethality in mice, (ii) NMDAR-mediated field excitatory postsynaptic potentials (fEPSPs) in the CA1 field of mouse hippocampal slices, (iii) NMDAR-mediated miniature excitatory postsynaptic currents (mEPSCs) and NMDA-evoked currents in CA1 pyramidal neurons of rat hippocampal slices, and (iv) recombinant NMDARs expressed in Xenopus oocytes. While a single i.p. injection of 10 mg/kg (2R,6R)-HNK exerted antidepressant-related behavioral and cellular responses in mice, the ED50 of (2R,6R)-HNK to prevent NMDA-induced lethality was found to be 228 mg/kg, compared with 6.4 mg/kg for ketamine. The 10 mg/kg (2R,6R)-HNK dose generated maximal hippocampal extracellular concentrations of ∼8 µM, which were well below concentrations required to inhibit synaptic and extrasynaptic NMDARs in vitro. (2S,6S)-HNK was more potent than (2R,6R)-HNK, but less potent than ketamine at inhibiting NMDARs. These data demonstrate the stereoselectivity of NMDAR inhibition by (2R,6R;2S,6S)-HNK and support the conclusion that direct NMDAR inhibition does not contribute to antidepressant-relevant effects of (2R,6R)-HNK.
Keywords: NMDA receptor; antidepressant; depression; hydroxynorketamine; ketamine.
Conflict of interest statement
Conflict of interest statement: T.D.G. has received research funding from Janssen, Roche, and Allergan Pharmaceuticals, and served as a consultant for FSV7 LLC during the preceding 3 years. The authors declare competing financial interests: T.D.G., P.Z., R.M., P.J.M., C.J.T., and C.A.Z. are listed as co-inventors on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. C.A.Z. and R.M. are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro- and hydroxylated metabolites of ketamine metabolites in the treatment of depression and neuropathic pain. R.M., P.J.M., C.J.T., and C.A.Z. have assigned patent rights to the US Government but will share a percentage of any royalties that may be received by the Government. P.Z. and T.D.G. have assigned their patent rights to the University of Maryland, Baltimore, but will share a percentage of any royalties that may be received by the University of Maryland, Baltimore. S.F.T. received research support from Janssen, is a consultant for Janssen, is a member of the Scientific Advisory Board for Sage Therapeutics, is a co-founder of NeurOp, Inc., and co-inventor on Emory-owned IP. S.J.M. owns stock in NeurOp, Inc., which is developing NMDAR inhibitors for use in treating neurological disease and disorders. All other authors declare no competing interests.
Figures

Similar articles
-
Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine.Neuropharmacology. 2019 Jul 15;153:73-81. doi: 10.1016/j.neuropharm.2019.04.019. Epub 2019 Apr 20. Neuropharmacology. 2019. PMID: 31015046
-
(R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses.Br J Pharmacol. 2019 Jul;176(14):2573-2592. doi: 10.1111/bph.14683. Epub 2019 May 13. Br J Pharmacol. 2019. PMID: 30941749 Free PMC article.
-
(2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6441-6450. doi: 10.1073/pnas.1819540116. Epub 2019 Mar 13. Proc Natl Acad Sci U S A. 2019. PMID: 30867285 Free PMC article.
-
Mechanisms of ketamine action as an antidepressant.Mol Psychiatry. 2018 Apr;23(4):801-811. doi: 10.1038/mp.2017.255. Epub 2018 Mar 13. Mol Psychiatry. 2018. PMID: 29532791 Free PMC article. Review.
-
CYP 450 enzymes influence (R,S)-ketamine brain delivery and its antidepressant activity.Neuropharmacology. 2022 Mar 15;206:108936. doi: 10.1016/j.neuropharm.2021.108936. Epub 2021 Dec 26. Neuropharmacology. 2022. PMID: 34965407 Review.
Cited by
-
(2R,6R)-hydroxynorketamine rapidly potentiates optically-evoked Schaffer collateral synaptic activity.Neuropharmacology. 2022 Aug 15;214:109153. doi: 10.1016/j.neuropharm.2022.109153. Epub 2022 May 31. Neuropharmacology. 2022. PMID: 35661657 Free PMC article.
-
(2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism.Neuropsychopharmacology. 2020 Jan;45(2):426-436. doi: 10.1038/s41386-019-0443-3. Epub 2019 Jun 19. Neuropsychopharmacology. 2020. PMID: 31216563 Free PMC article.
-
Metabonomic Profile and Signaling Pathway Prediction of Depression-Associated Suicidal Behavior.Front Psychiatry. 2020 Apr 16;11:269. doi: 10.3389/fpsyt.2020.00269. eCollection 2020. Front Psychiatry. 2020. PMID: 32372980 Free PMC article.
-
Ketamine and Attentional Bias Toward Emotional Faces: Dynamic Causal Modeling of Magnetoencephalographic Connectivity in Treatment-Resistant Depression.Front Psychiatry. 2021 Jun 18;12:673159. doi: 10.3389/fpsyt.2021.673159. eCollection 2021. Front Psychiatry. 2021. PMID: 34220581 Free PMC article.
-
Advancing past ketamine: emerging glutamatergic compounds for the treatment of depression.Eur Arch Psychiatry Clin Neurosci. 2024 Aug 29. doi: 10.1007/s00406-024-01875-z. Online ahead of print. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 39207462 Review.
References
-
- Kessler RC, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. - PubMed
-
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E. 2017 Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD), HHS Publication No. SMA 17-5044, NSDUH Series H-52. Available at https://www.samhsa.gov/data/. Accessed September 18 2018.
-
- Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. - PubMed
-
- Zarate CA, Jr, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
-
- Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

