FXR Regulates Intestinal Cancer Stem Cell Proliferation
- PMID: 30794774
- PMCID: PMC6701863
- DOI: 10.1016/j.cell.2019.01.036
FXR Regulates Intestinal Cancer Stem Cell Proliferation
Abstract
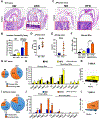
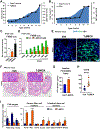
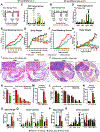
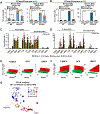
Increased levels of intestinal bile acids (BAs) are a risk factor for colorectal cancer (CRC). Here, we show that the convergence of dietary factors (high-fat diet) and dysregulated WNT signaling (APC mutation) alters BA profiles to drive malignant transformations in Lgr5-expressing (Lgr5+) cancer stem cells and promote an adenoma-to-adenocarcinoma progression. Mechanistically, we show that BAs that antagonize intestinal farnesoid X receptor (FXR) function, including tauro-β-muricholic acid (T-βMCA) and deoxycholic acid (DCA), induce proliferation and DNA damage in Lgr5+ cells. Conversely, selective activation of intestinal FXR can restrict abnormal Lgr5+ cell growth and curtail CRC progression. This unexpected role for FXR in coordinating intestinal self-renewal with BA levels implicates FXR as a potential therapeutic target for CRC.
Keywords: BA-FXR axis; Lgr5(+) intestinal stem cells; colon cancer progression; genetic and dietary risk factors.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
S.F., C.L., R.T.Y., A.R.A., M.D. and R.M.E. are co-inventors of inventions related to certain FXR agonists.
Figures



Similar articles
-
Bile acids and colon cancer: Is FXR the solution of the conundrum?Mol Aspects Med. 2017 Aug;56:66-74. doi: 10.1016/j.mam.2017.04.002. Epub 2017 Apr 21. Mol Aspects Med. 2017. PMID: 28400119 Review.
-
Microbial metabolite trimethylamine-N-oxide induces intestinal carcinogenesis through inhibiting farnesoid X receptor signaling.Cell Oncol (Dordr). 2024 Aug;47(4):1183-1199. doi: 10.1007/s13402-024-00920-2. Epub 2024 Feb 5. Cell Oncol (Dordr). 2024. PMID: 38315283
-
Inactivation of Adenomatous Polyposis Coli Reduces Bile Acid/Farnesoid X Receptor Expression through Fxr gene CpG Methylation in Mouse Colon Tumors and Human Colon Cancer Cells.J Nutr. 2016 Feb;146(2):236-42. doi: 10.3945/jn.115.216580. Epub 2015 Nov 25. J Nutr. 2016. PMID: 26609171 Free PMC article.
-
Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development.J Pharmacol Exp Ther. 2009 Feb;328(2):469-77. doi: 10.1124/jpet.108.145409. Epub 2008 Nov 3. J Pharmacol Exp Ther. 2009. PMID: 18981289 Free PMC article.
-
The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer.Nat Rev Gastroenterol Hepatol. 2021 May;18(5):335-347. doi: 10.1038/s41575-020-00404-2. Epub 2021 Feb 10. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33568795 Review.
Cited by
-
Targeting Lipid Metabolism in Cancer Stem Cells for Anticancer Treatment.Int J Mol Sci. 2024 Oct 17;25(20):11185. doi: 10.3390/ijms252011185. Int J Mol Sci. 2024. PMID: 39456967 Free PMC article. Review.
-
Effects of bile acids on the growth, composition and metabolism of gut bacteria.NPJ Biofilms Microbiomes. 2024 Oct 23;10(1):112. doi: 10.1038/s41522-024-00566-w. NPJ Biofilms Microbiomes. 2024. PMID: 39438471 Free PMC article.
-
Dietary and metabolic effects on intestinal stem cells in health and disease.Nat Rev Gastroenterol Hepatol. 2024 Oct 2. doi: 10.1038/s41575-024-00980-7. Online ahead of print. Nat Rev Gastroenterol Hepatol. 2024. PMID: 39358589 Review.
-
Secondary bile acids are associated with body lipid accumulation in obese pigs.Anim Nutr. 2024 Jun 22;18:246-256. doi: 10.1016/j.aninu.2024.04.019. eCollection 2024 Sep. Anim Nutr. 2024. PMID: 39281048 Free PMC article.
-
Nutrigenomic underpinnings of intestinal stem cells in inflammatory bowel disease and colorectal cancer development.Front Genet. 2024 Aug 30;15:1349717. doi: 10.3389/fgene.2024.1349717. eCollection 2024. Front Genet. 2024. PMID: 39280096 Free PMC article. Review.
References
-
- Aguirre-Gamboa R, Gomez-Rueda H, Martinez-Ledesma E, Martinez-Torteya A, Chacolla-Huaringa R, Rodriguez-Barrientos A, Tamez-Pena JG, and Trevino V (2013). SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS One 8, e74250. - PMC - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, and Clevers H (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

