The diagnostic accuracy of circulating free DNA for the detection of KRAS mutation status in colorectal cancer: A meta-analysis
- PMID: 30791218
- PMCID: PMC6434340
- DOI: 10.1002/cam4.1989
The diagnostic accuracy of circulating free DNA for the detection of KRAS mutation status in colorectal cancer: A meta-analysis
Abstract
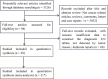
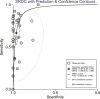
KRAS mutations have been reported as a reliable biomarker for epidermal growth factor receptor (EGFR) targeted therapy and are also associated with poor prognosis in colorectal cancer (CRC) patients. However, limitations of detecting KRAS mutations in tissues are obvious. KRAS mutations in the peripheral blood can be detected as an alternative to tissue analysis. The objective of this meta-analysis was to evaluate the diagnostic value of cfDNA (circulating free DNA) compared with tissues and to investigate the prognostic potential of cfDNA KRAS mutations in CRC patients. Searches were performed in PubMed, Embase, and Cochrane Library for published studies. We extracted true-positive (TP), false-positive (FP), false-negative (FN), true-negative (TN) values, survival rate of CRC patients with mutant and wild-type KRAS and calculated pooled sensitivity and specificity, positive/negative likelihood ratios [PLRs/NLRs], diagnostic odds ratios [DORs], and corresponding 95% confidence intervals [95% CIs]. We also generated a summary receiver operating characteristic (SROC) curve to evaluate the overall diagnostic potential. Totally, 31 relevant studies were recruited and used for the meta-analysis on the efficacy of cfDNA testing in detecting KRAS mutations. The pooled sensitivity, specificity, PLR, NLR, and DOR were 0.637 (95% CI: 0.607-0.666), 0.943 (95% CI: 0.930-0.954), 10.024 (95% CI: 6.912-14.535), 0.347 (95% CI: 0.269-0.447), and 37.882 (95% CI: 22.473-63.857), respectively. The area under the SROC curve was 0.9392. Together, the results suggest that detecting KRAS mutations in cfDNA has adequate diagnostic efficacy in terms of specificity. There is a promising role for cfDNA in the detection of KRAS mutations in CRC patients. However, prospective studies with larger patient cohorts are still required before definitive conclusions of the prognostic potential of cfDNA KRAS mutations in CRC patients were drawn.
Keywords: KRAS mutation; cfDNA; colorectal cancer; diagnostic; meta-analysis.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures




Similar articles
-
Diagnostic and prognostic value of blood samples for KRAS mutation identification in lung cancer: a meta-analysis.Oncotarget. 2017 May 30;8(22):36812-36823. doi: 10.18632/oncotarget.15972. Oncotarget. 2017. PMID: 28415658 Free PMC article. Review.
-
The diagnostic accuracy of digital PCR, ARMS and NGS for detecting KRAS mutation in cell-free DNA of patients with colorectal cancer: A systematic review and meta-analysis.PLoS One. 2021 Mar 26;16(3):e0248775. doi: 10.1371/journal.pone.0248775. eCollection 2021. PLoS One. 2021. PMID: 33770081 Free PMC article.
-
Detection of mutations in circulating cell-free DNA in relation to disease stage in colorectal cancer.Cancer Med. 2019 Jul;8(8):3761-3769. doi: 10.1002/cam4.2219. Epub 2019 May 27. Cancer Med. 2019. PMID: 31134762 Free PMC article.
-
Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis.Sci Rep. 2014 Sep 9;4:6269. doi: 10.1038/srep06269. Sci Rep. 2014. PMID: 25201768 Free PMC article. Review.
-
The potential of PIK3CA, KRAS, BRAF, and APC hotspot mutations as a non-invasive detection method for colorectal cancer.Mol Cell Probes. 2022 Jun;63:101807. doi: 10.1016/j.mcp.2022.101807. Epub 2022 Mar 13. Mol Cell Probes. 2022. PMID: 35296442
Cited by
-
The Interaction Between Epigenetic Changes, EMT, and Exosomes in Predicting Metastasis of Colorectal Cancers (CRC).Front Oncol. 2022 May 30;12:879848. doi: 10.3389/fonc.2022.879848. eCollection 2022. Front Oncol. 2022. PMID: 35712512 Free PMC article. Review.
-
Towards Circulating-Tumor DNA-Based Precision Medicine.J Clin Med. 2019 Sep 2;8(9):1365. doi: 10.3390/jcm8091365. J Clin Med. 2019. PMID: 31480647 Free PMC article. Review.
-
Diagnostic value of liquid biopsy in the era of precision medicine: 10 years of clinical evidence in cancer.Explor Target Antitumor Ther. 2023;4(1):102-138. doi: 10.37349/etat.2023.00125. Epub 2023 Feb 28. Explor Target Antitumor Ther. 2023. PMID: 36937316 Free PMC article. Review.
-
Predicting Colorectal Cancer Occurrence in IBD.Cancers (Basel). 2021 Jun 10;13(12):2908. doi: 10.3390/cancers13122908. Cancers (Basel). 2021. PMID: 34200768 Free PMC article. Review.
-
PNA Clamping in Nucleic Acid Amplification Protocols to Detect Single Nucleotide Mutations Related to Cancer.Molecules. 2020 Feb 12;25(4):786. doi: 10.3390/molecules25040786. Molecules. 2020. PMID: 32059456 Free PMC article. Review.
References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87‐108. - PubMed
-
- Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992‐3995. - PubMed
-
- Qiu LX, Mao C, Zhang J, et al. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta‐analysis of 22 studies. Eur J Cancer. 2010;46:2781‐2787. - PubMed
-
- Amado RG, Wolf M, Peeters M, et al. Wild‐type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626‐1634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

