Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca2+-ATPase in Heart Failure
- PMID: 30786847
- PMCID: PMC6483854
- DOI: 10.1161/CIRCRESAHA.118.313865
Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca2+-ATPase in Heart Failure
Erratum in
-
Correction to: Role of SIRT1 in Modulating Acetylation of the Sarco- Endoplasmic Reticulum Ca2+-ATPase in Heart Failure.Circ Res. 2019 Jun 7;124(12):e149. doi: 10.1161/RES.0000000000000277. Epub 2019 Jun 6. Circ Res. 2019. PMID: 31170043 No abstract available.
Abstract
Rationale: SERCA2a, sarco-endoplasmic reticulum Ca2+-ATPase, is a critical determinant of cardiac function. Reduced level and activity of SERCA2a are major features of heart failure. Accordingly, intensive efforts have been made to develop efficient modalities for SERCA2a activation. We showed that the activity of SERCA2a is enhanced by post-translational modification with SUMO1 (small ubiquitin-like modifier 1). However, the roles of other post-translational modifications on SERCA2a are still unknown.
Objective: In this study, we aim to assess the role of lysine acetylation on SERCA2a function and determine whether inhibition of lysine acetylation can improve cardiac function in the setting of heart failure.
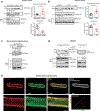
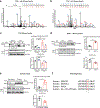
Methods and results: The acetylation of SERCA2a was significantly increased in failing hearts of humans, mice, and pigs, which is associated with the reduced level of SIRT1 (sirtuin 1), a class III histone deacetylase. Downregulation of SIRT1 increased the SERCA2a acetylation, which in turn led to SERCA2a dysfunction and cardiac defects at baseline. In contrast, pharmacological activation of SIRT1 reduced the SERCA2a acetylation, which was accompanied by recovery of SERCA2a function and cardiac defects in failing hearts. Lysine 492 (K492) was of critical importance for the regulation of SERCA2a activity via acetylation. Acetylation at K492 significantly reduced the SERCA2a activity, presumably through interfering with the binding of ATP to SERCA2a. In failing hearts, acetylation at K492 appeared to be mediated by p300 (histone acetyltransferase p300), a histone acetyltransferase.
Conclusions: These results indicate that acetylation/deacetylation at K492, which is regulated by SIRT1 and p300, is critical for the regulation of SERCA2a activity in hearts. Pharmacological activation of SIRT1 can restore SERCA2a activity through deacetylation at K492. These findings might provide a novel strategy for the treatment of heart failure.
Keywords: acetylation; endoplasmic reticulum; heart failure; lysine; mice.
Figures






Comment in
-
Acetylation of SERCA2a, Another Target for Heart Failure Treatment?Circ Res. 2019 Apr 26;124(9):1285-1287. doi: 10.1161/CIRCRESAHA.119.315017. Circ Res. 2019. PMID: 31021723 Free PMC article. No abstract available.
Similar articles
-
Identification and Characterization of p300-Mediated Lysine Residues in Cardiac SERCA2a.Int J Mol Sci. 2023 Feb 9;24(4):3502. doi: 10.3390/ijms24043502. Int J Mol Sci. 2023. PMID: 36834924 Free PMC article.
-
SUMO1-dependent modulation of SERCA2a in heart failure.Nature. 2011 Sep 7;477(7366):601-5. doi: 10.1038/nature10407. Nature. 2011. PMID: 21900893 Free PMC article.
-
Muscle-specific sirtuin 3 overexpression does not attenuate the pathological effects of high-fat/high-sucrose feeding but does enhance cardiac SERCA2a activity.Physiol Rep. 2021 Aug;9(16):e14961. doi: 10.14814/phy2.14961. Physiol Rep. 2021. PMID: 34405591 Free PMC article.
-
[Modification of sarco-endoplasmic reticulum Ca(2 +) -ATPase in the failing cardiomyocyte].Clin Calcium. 2013 Apr;23(4):535-42. Clin Calcium. 2013. PMID: 23545743 Review. Japanese.
-
SERCA2a: a prime target for modulation of cardiac contractility during heart failure.BMB Rep. 2013 May;46(5):237-43. doi: 10.5483/bmbrep.2013.46.5.077. BMB Rep. 2013. PMID: 23710633 Free PMC article. Review.
Cited by
-
Screening of key genes related to M6A methylation in patients with heart failure.BMC Cardiovasc Disord. 2024 Oct 16;24(1):565. doi: 10.1186/s12872-024-04228-9. BMC Cardiovasc Disord. 2024. PMID: 39415091 Free PMC article.
-
Inducible Cardiac-Specific Deletion of Sirt1 in Male Mice Reveals Progressive Cardiac Dysfunction and Sensitization of the Heart to Pressure Overload.Int J Mol Sci. 2019 Oct 10;20(20):5005. doi: 10.3390/ijms20205005. Int J Mol Sci. 2019. PMID: 31658614 Free PMC article.
-
LncRNA CCRR maintains Ca2+ homeostasis against myocardial infarction through the FTO-SERCA2a pathway.Sci China Life Sci. 2024 Aug;67(8):1601-1619. doi: 10.1007/s11427-023-2527-5. Epub 2024 May 16. Sci China Life Sci. 2024. PMID: 38761356
-
Chronic SIRT1 supplementation in diabetic mice improves endothelial function by suppressing oxidative stress.Cardiovasc Res. 2023 Oct 16;119(12):2190-2201. doi: 10.1093/cvr/cvad102. Cardiovasc Res. 2023. PMID: 37401647 Free PMC article.
-
Shaping cardiac destiny: the role of post-translational modifications on endoplasmic reticulum - mitochondria crosstalk in cardiac remodeling.Front Pharmacol. 2024 Oct 11;15:1423356. doi: 10.3389/fphar.2024.1423356. eCollection 2024. Front Pharmacol. 2024. PMID: 39464632 Free PMC article. Review.
References
-
- Kawase Y and Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–65. - PubMed
-
- Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Takewa Y, Jeong D, Park WJ, Kawase Y and Hajjar RJ. Targeted gene transfer increases contractility and decreases oxygen cost of contractility in normal rat hearts. American journal of physiology Heart and circulatory physiology. 2007;292:H2356–63. - PubMed
-
- Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A and Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:793–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 17POST33410877/AHA/American Heart Association-American Stroke Association/United States
- R01 HL131404/HL/NHLBI NIH HHS/United States
- P50 HL112324/HL/NHLBI NIH HHS/United States
- R01 HL119046/HL/NHLBI NIH HHS/United States
- R01 HL128099/HL/NHLBI NIH HHS/United States
- R01 HL117505/HL/NHLBI NIH HHS/United States
- CIHR/Canada
- T32 HL007824/HL/NHLBI NIH HHS/United States
- R01 HL129814/HL/NHLBI NIH HHS/United States
- R42 HL132684/HL/NHLBI NIH HHS/United States
- R00 HL116645/HL/NHLBI NIH HHS/United States
- 18TPA34170460/AHA/American Heart Association-American Stroke Association/United States
- K99 HL116645/HL/NHLBI NIH HHS/United States
- R43 HL144223/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

