Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis
- PMID: 30786740
- PMCID: PMC6482082
- DOI: 10.1161/ATVBAHA.119.312434
Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis
Abstract
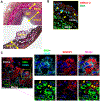
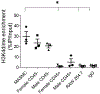
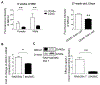
Objective- Smooth muscle cells (SMCs) are the most abundant cells in human atherosclerotic lesions and are suggested to contribute at least 50% of atheroma foam cells. In mice, SMCs contribute fewer total lesional cells. The purpose of this study was to determine the contribution of SMCs to total foam cells in apolipoprotein E-deficient (ApoE-/-) mice, and the utility of these mice to model human SMC foam cell biology and interventions. Approach and Results- Using flow cytometry, foam cells in the aortic arch of ApoE-/- mice were characterized based on the expression of leukocyte-specific markers. Nonleukocyte foam cells increased from 37% of total foam cells in 27-week-old to 75% in 57-week-old male ApoE-/- mice fed a chow diet and were ≈70% in male and female ApoE-/- mice following 6 weeks of Western diet feeding. A similar contribution to total foam cells by SMCs was found using SMC-lineage tracing ApoE-/- mice fed the Western diet for 6 or 12 weeks. Nonleukocyte foam cells contributed a similar percentage of total atheroma cholesterol and exhibited lower expression of the cholesterol exporter ABCA1 (ATP-binding cassette transporter A1) when compared with leukocyte-derived foam cells. Conclusions- Consistent with previous studies of human atheromas, we present evidence that SMCs contribute the majority of atheroma foam cells in ApoE-/- mice fed a Western diet and a chow diet for longer periods. Reduced expression of ABCA1, also seen in human intimal SMCs, suggests a common mechanism for formation of SMC foam cells across species, and represents a novel target to enhance atherosclerosis regression.
Keywords: atherosclerosis; cholesterol; foam cells; macrophages; smooth muscle.
Conflict of interest statement
Disclosures: None
Figures


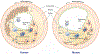
Comment in
-
Revealing the Origins of Foam Cells in Atherosclerotic Lesions.Arterioscler Thromb Vasc Biol. 2019 May;39(5):836-838. doi: 10.1161/ATVBAHA.119.312557. Arterioscler Thromb Vasc Biol. 2019. PMID: 31017823 Free PMC article. No abstract available.
Similar articles
-
Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis.Circulation. 2014 Apr 15;129(15):1551-9. doi: 10.1161/CIRCULATIONAHA.113.005015. Epub 2014 Jan 30. Circulation. 2014. PMID: 24481950
-
Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation.Cardiovasc Res. 2012 Jul 15;95(2):165-72. doi: 10.1093/cvr/cvs094. Epub 2012 Feb 15. Cardiovasc Res. 2012. PMID: 22345306 Review.
-
Thrombin-Par1 signaling axis disrupts COP9 signalosome subunit 3-mediated ABCA1 stabilization in inducing foam cell formation and atherogenesis.Cell Death Differ. 2021 Feb;28(2):780-798. doi: 10.1038/s41418-020-00623-9. Epub 2020 Sep 23. Cell Death Differ. 2021. PMID: 32968199 Free PMC article.
-
Cholesterol homeostasis and high-density lipoprotein formation in arterial smooth muscle cells.Trends Cardiovasc Med. 2010 Apr;20(3):96-102. doi: 10.1016/j.tcm.2010.09.002. Trends Cardiovasc Med. 2010. PMID: 21130953 Review.
-
ATP-binding cassette transporter A1 expression and apolipoprotein A-I binding are impaired in intima-type arterial smooth muscle cells.Circulation. 2009 Jun 30;119(25):3223-31. doi: 10.1161/CIRCULATIONAHA.108.841130. Epub 2009 Jun 15. Circulation. 2009. PMID: 19528336
Cited by
-
Insights from Murine Studies on the Site Specificity of Atherosclerosis.Int J Mol Sci. 2024 Jun 9;25(12):6375. doi: 10.3390/ijms25126375. Int J Mol Sci. 2024. PMID: 38928086 Free PMC article. Review.
-
Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis.Circulation. 2020 Nov 24;142(21):2045-2059. doi: 10.1161/CIRCULATIONAHA.120.046672. Epub 2020 Jul 17. Circulation. 2020. PMID: 32674599 Free PMC article.
-
Top Five Stories of the Cellular Landscape and Therapies of Atherosclerosis: Current Knowledge and Future Perspectives.Curr Med Sci. 2024 Feb;44(1):1-27. doi: 10.1007/s11596-023-2818-2. Epub 2023 Dec 7. Curr Med Sci. 2024. PMID: 38057537 Review.
-
Molecular Interactions Between Vascular Smooth Muscle Cells and Macrophages in Atherosclerosis.Front Cardiovasc Med. 2021 Oct 15;8:737934. doi: 10.3389/fcvm.2021.737934. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34722670 Free PMC article.
-
Dynamic changes in chromatin accessibility are associated with the atherogenic transitioning of vascular smooth muscle cells.Cardiovasc Res. 2022 Oct 21;118(13):2792-2804. doi: 10.1093/cvr/cvab347. Cardiovasc Res. 2022. PMID: 34849613 Free PMC article.
References
-
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844 - PubMed
-
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

