Enterovirus A71 vaccine effectiveness in preventing enterovirus A71 infection among medically-attended hand, foot, and mouth disease cases, Beijing, China
- PMID: 30779680
- PMCID: PMC6605830
- DOI: 10.1080/21645515.2019.1581539
Enterovirus A71 vaccine effectiveness in preventing enterovirus A71 infection among medically-attended hand, foot, and mouth disease cases, Beijing, China
Abstract
Introduction: Enterovirus A71(EV-A71)-associated hand, foot, and mouth disease (HFMD) has been reported worldwide, and poses a particularly heavy burden on patients, families, and society in China. Three Chinese companies have licensed inactivated EV-A71 vaccines, all of which have demonstrated good efficacy for preventing EV-A71-associated disease in clinical trials. However, real-world performance of EV-A71 vaccine has not been evaluated.
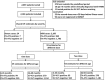
Methods: We used a test-negative design case-control study to estimate vaccine effectiveness (VE) against medically attended EV-A71-associated HFMD. Subjects were children 5 years of age and under who had been in health facilities participating in the HFMD case and virologic surveillance platforms in Beijing. Enterovirus infections were laboratory confirmed, and EV-A71 vaccination status was extracted from electronic immunization records. Children testing positive for EV-A71 were cases; controls were children testing negative for EV-A71 infection. Logistic regression was used to estimate VE. We assessed sensitivity of VE estimates to control group inclusion criteria by repeating the regression analyses with two alternative control groups.
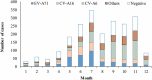
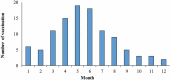
Results: A total of 2,184 HFMD patients aged 5 years and under were enrolled in the study; 24 were severe, and 2,160 were mild. For severe cases, two-dose VE estimate was 100% (95% CI: -68.1%, 100%). For mild cases, 1-dose and 2-dose adjusted VE estimates were 69.8% and 83.7%, respectively. Two-dose VE estimates varied by less than 4 percentage points regardless of control group definition.
Conclusions: Our findings suggested the vaccines performed well in the real world for children 5 years of age and under in Beijing, China.
Keywords: Hand; and mouth disease; enterovirus infections; foot; inactivated vaccines.
Figures

Similar articles
-
Effectiveness of enterovirus A71 vaccine in severe hand, foot, and mouth disease cases in Guangxi, China.Vaccine. 2020 Feb 11;38(7):1804-1809. doi: 10.1016/j.vaccine.2019.12.025. Epub 2019 Dec 28. Vaccine. 2020. PMID: 31892446
-
Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017-18: a test-negative case-control study.Lancet Child Adolesc Health. 2019 Oct;3(10):697-704. doi: 10.1016/S2352-4642(19)30185-3. Epub 2019 Jul 30. Lancet Child Adolesc Health. 2019. PMID: 31375313 Free PMC article.
-
Molecular epidemiology and clinical features of hand, foot and mouth disease requiring hospitalization after the use of enterovirus A71 inactivated vaccine in chengdu, China, 2017-2022: a descriptive study.Emerg Microbes Infect. 2022 Dec;11(1):2510-2519. doi: 10.1080/22221751.2022.2125346. Emerg Microbes Infect. 2022. PMID: 36103331 Free PMC article.
-
Is a multivalent hand, foot, and mouth disease vaccine feasible?Hum Vaccin Immunother. 2015;11(11):2688-704. doi: 10.1080/21645515.2015.1049780. Epub 2015 May 26. Hum Vaccin Immunother. 2015. PMID: 26009802 Free PMC article. Review.
-
Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease.Expert Rev Vaccines. 2018 Sep;17(9):819-831. doi: 10.1080/14760584.2018.1510326. Epub 2018 Aug 22. Expert Rev Vaccines. 2018. PMID: 30095317 Review.
Cited by
-
Spatiotemporal cluster patterns of hand, foot, and mouth disease at the province level in mainland China, 2011-2018.PLoS One. 2022 Aug 22;17(8):e0270061. doi: 10.1371/journal.pone.0270061. eCollection 2022. PLoS One. 2022. PMID: 35994464 Free PMC article.
-
An epidemiological surveillance of hand foot and mouth disease in paediatric patients and in community: A Singapore retrospective cohort study, 2013-2018.PLoS Negl Trop Dis. 2021 Feb 10;15(2):e0008885. doi: 10.1371/journal.pntd.0008885. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33566802 Free PMC article.
-
Epidemiology and etiology of hand, foot, and mouth disease in Zhengzhou, China, from 2009 to 2021.Infect Med (Beijing). 2024 Apr 20;3(2):100114. doi: 10.1016/j.imj.2024.100114. eCollection 2024 Jun. Infect Med (Beijing). 2024. PMID: 38974346 Free PMC article.
-
Progress in research and development of preventive vaccines for children in China.Front Pediatr. 2024 Jul 3;12:1414177. doi: 10.3389/fped.2024.1414177. eCollection 2024. Front Pediatr. 2024. PMID: 39022216 Free PMC article. Review.
-
Enterovirus A71 and coxsackievirus A6 circulation in England, UK, 2006-2017: A mathematical modelling study using cross-sectional seroprevalence data.PLoS Pathog. 2024 Nov 20;20(11):e1012703. doi: 10.1371/journal.ppat.1012703. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39565769 Free PMC article.
References
-
- Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, Ho KK, Han LL, Pallansch MA, Suleiman AB, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the outbreak study group. Clin Infect Dis. 2000;31:678–83. doi:10.1086/314032. - DOI - PubMed
-
- Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, Totsuka A, Nishio O.. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol. 2002;46:621–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
