A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs
- PMID: 30774999
- PMCID: PMC6368541
- DOI: 10.1038/s41541-019-0104-x
A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs
Abstract
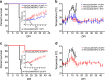
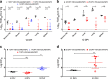
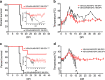
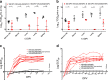
The World Health Organization has identified Lassa virus (LASV) as one of the top five pathogens to cause a severe outbreak in the near future. This study assesses the ability of a leading vaccine candidate, recombinant Vesicular stomatitis virus expressing LASV glycoprotein (VSVΔG/LASVGPC), and its ability to induce rapid and long-term immunity to lethal guinea pig-adapted LASV (GPA-LASV). Outbred guinea pigs were vaccinated with a single dose of VSVΔG/LASVGPC followed by a lethal challenge of GPA-LASV at 7, 14, 25, 189, and 355 days post-vaccination. Statistically significant rapid and long-term protection was achieved at all time points with 100% protection at days 7 and 14 post-vaccination. While 83 and 87% protection were achieved at 25 days and 6 months post-vaccination, respectively. When guinea pigs were challenged one year after vaccination 71% protection was achieved. Notable infectious virus was isolated from the serum and tissues of some but not all animals. Total LASVGPC-specific IgG titers were also measured on a monthly basis leading up to LASV challenge however, it is unclear if antibody alone correlates with short and long term survival. These studies confirm that a single dose of VSVΔG/LASVGPC can induce rapid and long-term protection from LASV infection in an aggressive outbred model of infection, and supports further development in non-human primates.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses.PLoS Negl Trop Dis. 2015 Apr 17;9(4):e0003736. doi: 10.1371/journal.pntd.0003736. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25884628 Free PMC article.
-
A recombinant VSV-vectored vaccine rapidly protects nonhuman primates against heterologous lethal Lassa fever.Cell Rep. 2022 Jul 19;40(3):111094. doi: 10.1016/j.celrep.2022.111094. Cell Rep. 2022. PMID: 35858566
-
ChAdOx1-vectored Lassa fever vaccine elicits a robust cellular and humoral immune response and protects guinea pigs against lethal Lassa virus challenge.NPJ Vaccines. 2021 Mar 2;6(1):32. doi: 10.1038/s41541-021-00291-x. NPJ Vaccines. 2021. PMID: 33654106 Free PMC article.
-
Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development.NPJ Vaccines. 2018 Mar 20;3:11. doi: 10.1038/s41541-018-0049-5. eCollection 2018. NPJ Vaccines. 2018. PMID: 29581897 Free PMC article. Review.
-
Vaccine platforms to control Lassa fever.Expert Rev Vaccines. 2016 Sep;15(9):1135-50. doi: 10.1080/14760584.2016.1184575. Epub 2016 May 24. Expert Rev Vaccines. 2016. PMID: 27136941 Review.
Cited by
-
Pathogen Dose in Animal Models of Hemorrhagic Fever Virus Infections and the Potential Impact on Studies of the Immune Response.Pathogens. 2021 Mar 1;10(3):275. doi: 10.3390/pathogens10030275. Pathogens. 2021. PMID: 33804381 Free PMC article. Review.
-
Vesicular Stomatitis Virus-Based Vaccines Provide Cross-Protection against Andes and Sin Nombre Viruses.Viruses. 2019 Jul 13;11(7):645. doi: 10.3390/v11070645. Viruses. 2019. PMID: 31337019 Free PMC article.
-
Defining bottlenecks and opportunities for Lassa virus neutralization by structural profiling of vaccine-induced polyclonal antibody responses.Cell Rep. 2024 Sep 24;43(9):114708. doi: 10.1016/j.celrep.2024.114708. Epub 2024 Sep 6. Cell Rep. 2024. PMID: 39243373 Free PMC article.
-
Adjuvants Differentially Modulate the Immunogenicity of Lassa Virus Glycoprotein Subunits in Mice.Front Trop Dis. 2022;3:847598. doi: 10.3389/fitd.2022.847598. Epub 2022 Mar 10. Front Trop Dis. 2022. PMID: 37034031 Free PMC article.
-
Production and characterization of lentivirus vector-based SARS-CoV-2 pseudoviruses with dual reporters: Evaluation of anti-SARS-CoV-2 viral effect of Korean Red Ginseng.J Ginseng Res. 2023 Jan;47(1):123-132. doi: 10.1016/j.jgr.2022.07.003. Epub 2022 Jul 15. J Ginseng Res. 2023. PMID: 35855181 Free PMC article.
References
-
- Ogbu, O., Ajuluchukwu, E. & Uneke, C. J. Lassa fever in West African sub-region: an overview. J Vector Borne44, 1–11 (2007). - PubMed
LinkOut - more resources
Full Text Sources

