Transcription Factor EB Activation Rescues Advanced αB-Crystallin Mutation-Induced Cardiomyopathy by Normalizing Desmin Localization
- PMID: 30773991
- PMCID: PMC6405666
- DOI: 10.1161/JAHA.118.010866
Transcription Factor EB Activation Rescues Advanced αB-Crystallin Mutation-Induced Cardiomyopathy by Normalizing Desmin Localization
Abstract
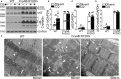
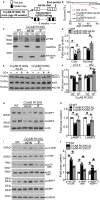
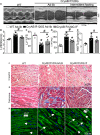
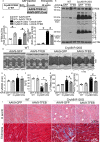
Background Mutations in αB-crystallin result in proteotoxic cardiomyopathy with desmin mislocalization to protein aggregates. Intermittent fasting ( IF ) is a novel approach to activate transcription factor EB (TFEB), a master regulator of the autophagy-lysosomal pathway, in the myocardium. We tested whether TFEB activation can be harnessed to treat advanced proteotoxic cardiomyopathy. Methods and Results Mice overexpressing the R120G mutant of αB-crystallin in cardiomyocytes ( Myh6-Cry ABR 120G) were subjected to IF or ad-lib feeding, or transduced with adeno-associated virus- TFEB or adeno-associated virus-green fluorescent protein after development of advanced proteotoxic cardiomyopathy. Adeno-associated virus-short hairpin RNA-mediated knockdown of TFEB and HSPB 8 was performed simultaneously with IF . Myh6-Cry ABR 120G mice demonstrated impaired autophagic flux, reduced lysosome abundance, and mammalian target of rapamycin activation in the myocardium. IF resulted in mammalian target of rapamycin inhibition and nuclear translocation of TFEB with restored lysosome abundance and autophagic flux; and reduced aggregates with normalized desmin localization. IF also attenuated left ventricular dilation and myocardial hypertrophy, increased percentage fractional shortening, and increased survival. Adeno-associated virus- TFEB transduction was sufficient to rescue cardiomyopathic manifestations, and resulted in reduced aggregates and normalized desmin localization in Myh6-Cry ABR 120G mice. Cry ABR 120G-expressing hearts demonstrated increased interaction of desmin with αB-crystallin and reduced interaction with chaperone protein, HSPB 8, compared with wild type, which was reversed by both IF and TFEB transduction. TFEB stimulated autophagic flux to remove protein aggregates and transcriptionally upregulated HSPB 8, to restore normal desmin localization in Cry ABR 120G-expressing cardiomyocytes. Short hairpin RNA-mediated knockdown of TFEB and HSPB 8 abrogated IF effects, in vivo. Conclusions IF and TFEB activation are clinically relevant therapeutic strategies to rescue advanced R120G αB-crystallin mutant-induced cardiomyopathy by normalizing desmin localization via autophagy-dependent and autophagy-independent mechanisms.
Keywords: HspB8; TFEB; cardiomyopathy; intermittent fasting; protein aggregates; αB‐crystallin.
Figures

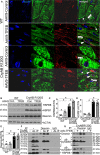
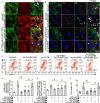
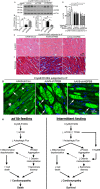
Comment in
-
Intermittent Fasting Reverses an Advanced Form of Cardiomyopathy.J Am Heart Assoc. 2019 Feb 19;8(4):e011863. doi: 10.1161/JAHA.118.011863. J Am Heart Assoc. 2019. PMID: 30773085 Free PMC article.
Similar articles
-
TFEB activation protects against cardiac proteotoxicity via increasing autophagic flux.J Mol Cell Cardiol. 2017 Dec;113:51-62. doi: 10.1016/j.yjmcc.2017.10.003. Epub 2017 Oct 7. J Mol Cell Cardiol. 2017. PMID: 28993153 Free PMC article.
-
Inhibition of Mutant αB Crystallin-Induced Protein Aggregation by a Molecular Tweezer.J Am Heart Assoc. 2017 Aug 8;6(8):e006182. doi: 10.1161/JAHA.117.006182. J Am Heart Assoc. 2017. PMID: 28862927 Free PMC article.
-
Autophagy and p62 in cardiac proteinopathy.Circ Res. 2011 Jul 22;109(3):296-308. doi: 10.1161/CIRCRESAHA.111.244707. Epub 2011 Jun 9. Circ Res. 2011. PMID: 21659648 Free PMC article.
-
Desmin filaments and cardiac disease: establishing causality.J Card Fail. 2002 Dec;8(6 Suppl):S287-92. doi: 10.1054/jcaf.2002.129279. J Card Fail. 2002. PMID: 12555134 Review.
-
Ragopathies and the rising influence of RagGTPases on human diseases.Nat Commun. 2024 Jul 10;15(1):5812. doi: 10.1038/s41467-024-50034-4. Nat Commun. 2024. PMID: 38987251 Free PMC article. Review.
Cited by
-
M6A modification in cardiovascular disease: With a focus on programmed cell death.Genes Dis. 2023 Jul 14;11(5):101039. doi: 10.1016/j.gendis.2023.05.023. eCollection 2024 Sep. Genes Dis. 2023. PMID: 38988324 Free PMC article. Review.
-
Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity.Cell Metab. 2023 Jun 6;35(6):928-942.e4. doi: 10.1016/j.cmet.2023.02.006. Epub 2023 Mar 2. Cell Metab. 2023. PMID: 36868222 Free PMC article. Clinical Trial.
-
Fueling the Heart: What Are the Optimal Dietary Strategies in Heart Failure?Nutrients. 2024 Sep 18;16(18):3157. doi: 10.3390/nu16183157. Nutrients. 2024. PMID: 39339757 Free PMC article. Review.
-
Targeting the Autophagy-Lysosome Pathway in a Pathophysiologically Relevant Murine Model of Reversible Heart Failure.JACC Basic Transl Sci. 2022 Oct 19;7(12):1214-1228. doi: 10.1016/j.jacbts.2022.06.003. eCollection 2022 Dec. JACC Basic Transl Sci. 2022. PMID: 36644282 Free PMC article.
-
NO to Lysosomes: A Signal for Insulin Resistance in Obesity.Cell Mol Gastroenterol Hepatol. 2019;8(1):153-154. doi: 10.1016/j.jcmgh.2019.04.006. Epub 2019 May 15. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31102588 Free PMC article. No abstract available.
References
-
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB‐crystallin chaperone gene causes a desmin‐related myopathy. Nat Genet. 1998;20:92–95. - PubMed
-
- Goldfarb LG, Park KY, Cervenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle JW, Semino‐Mora C, Sivakumar K, Dalakas MC. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet. 1998;19:402–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

