The cardiomyocyte "redox rheostat": Redox signalling via the AMPK-mTOR axis and regulation of gene and protein expression balancing survival and death
- PMID: 30771309
- PMCID: PMC6497135
- DOI: 10.1016/j.yjmcc.2019.02.006
The cardiomyocyte "redox rheostat": Redox signalling via the AMPK-mTOR axis and regulation of gene and protein expression balancing survival and death
Abstract
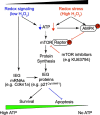
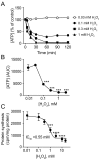
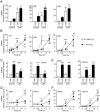
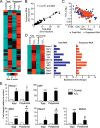
Reactive oxygen species (ROS) play a key role in development of heart failure but, at a cellular level, their effects range from cytoprotection to induction of cell death. Understanding how this is regulated is crucial to develop novel strategies to ameliorate only the detrimental effects. Here, we revisited the fundamental hypothesis that the level of ROS per se is a key factor in the cellular response by applying different concentrations of H2O2 to cardiomyocytes. High concentrations rapidly reduced intracellular ATP and inhibited protein synthesis. This was associated with activation of AMPK which phosphorylated and inhibited Raptor, a crucial component of mTOR complex-1 that regulates protein synthesis. Inhibition of protein synthesis by high concentrations of H2O2 prevents synthesis of immediate early gene products required for downstream gene expression, and such mRNAs (many encoding proteins required to deal with oxidant stress) were only induced by lower concentrations. Lower concentrations of H2O2 promoted mTOR phosphorylation, associated with differential recruitment of some mRNAs to the polysomes for translation. Some of the upregulated genes induced by low H2O2 levels are cytoprotective. We identified p21Cip1/WAF1 as one such protein, and preventing its upregulation enhanced the rate of cardiomyocyte apoptosis. The data support the concept of a "redox rheostat" in which different degrees of ROS influence cell energetics and intracellular signalling pathways to regulate mRNA and protein expression. This sliding scale determines cell fate, modulating survival vs death.
Keywords: Cytoprotection; Immediate early genes; Oxidative stress; Protein synthesis; Raptor; mTOR; p21(Cip1/WAF1).
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Adiponectin modulates oxidative stress-induced autophagy in cardiomyocytes.PLoS One. 2013 Jul 19;8(7):e68697. doi: 10.1371/journal.pone.0068697. Print 2013. PLoS One. 2013. PMID: 23894332 Free PMC article.
-
Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways.Cell Physiol Biochem. 2017;43(1):52-68. doi: 10.1159/000480317. Epub 2017 Aug 25. Cell Physiol Biochem. 2017. PMID: 28848091
-
Inhibition of AMPK signalling by doxorubicin: at the crossroads of the cardiac responses to energetic, oxidative, and genotoxic stress.Cardiovasc Res. 2012 Aug 1;95(3):290-9. doi: 10.1093/cvr/cvs134. Epub 2012 Mar 28. Cardiovasc Res. 2012. PMID: 22461523
-
LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review.
-
Effects of Oxidative Stress on Protein Translation: Implications for Cardiovascular Diseases.Int J Mol Sci. 2020 Apr 11;21(8):2661. doi: 10.3390/ijms21082661. Int J Mol Sci. 2020. PMID: 32290431 Free PMC article. Review.
Cited by
-
Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases.Antioxidants (Basel). 2021 Jul 22;10(8):1166. doi: 10.3390/antiox10081166. Antioxidants (Basel). 2021. PMID: 34439414 Free PMC article. Review.
-
Redox Regulation of Cardiac ASK1 (Apoptosis Signal-Regulating Kinase 1) Controls p38-MAPK (Mitogen-Activated Protein Kinase) and Orchestrates Cardiac Remodeling to Hypertension.Hypertension. 2020 Oct;76(4):1208-1218. doi: 10.1161/HYPERTENSIONAHA.119.14556. Epub 2020 Sep 9. Hypertension. 2020. PMID: 32903101 Free PMC article.
-
NSAIDs-dependent adaption of the mitochondria-proteasome system in immortalized human cardiomyocytes.Sci Rep. 2020 Oct 27;10(1):18337. doi: 10.1038/s41598-020-75394-x. Sci Rep. 2020. PMID: 33110169 Free PMC article.
-
Autophagy and Redox Homeostasis in Parkinson's: A Crucial Balancing Act.Oxid Med Cell Longev. 2020 Nov 10;2020:8865611. doi: 10.1155/2020/8865611. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33224433 Free PMC article. Review.
-
Dietary AGEs involvement in colonic inflammation and cancer: insights from an in vitro enterocyte model.Sci Rep. 2020 Feb 17;10(1):2754. doi: 10.1038/s41598-020-59623-x. Sci Rep. 2020. PMID: 32066788 Free PMC article.
References
-
- Metra M., Teerlink J.R. Heart failure. Lancet. 2017;390:1981–1995. - PubMed
-
- Takemura G., Kanoh M., Minatoguchi S., Fujiwara H. Cardiomyocyte apoptosis in the failing heart—a critical review from definition and classification of cell death. Int. J. Cardiol. 2013;167:2373–2386. - PubMed
-
- Zhang J., Liu D., Zhang M., Zhang Y. Programmed necrosis in cardiomyocytes: mitochondria, death receptors and beyond. Br. J. Pharmacol. 2018 https://www.ncbi.nlm.nih.gov/pubmed/29774530 (E-pub ahead of print) - PMC - PubMed
-
- Sugden P.H., Clerk A. Oxidative stress and growth-regulating intracellular signalling pathways in cardiac myocytes. Antioxid. Redox. Signal. 2006;8:2111–2124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

