PGC1A regulates the IRS1:IRS2 ratio during fasting to influence hepatic metabolism downstream of insulin
- PMID: 30770439
- PMCID: PMC6410797
- DOI: 10.1073/pnas.1815150116
PGC1A regulates the IRS1:IRS2 ratio during fasting to influence hepatic metabolism downstream of insulin
Abstract
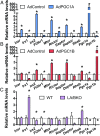
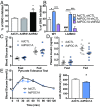
Precise modulation of hepatic glucose metabolism is crucial during the fasting and feeding cycle and is controlled by the actions of circulating insulin and glucagon. The insulin-signaling pathway requires insulin receptor substrate 1 (IRS1) and IRS2, which are found to be dysregulated in diabetes and obesity. The peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1A) is a fasting-induced transcriptional coactivator. In nonalcoholic fatty liver disease and in patients with type 2 diabetes, low hepatic PGC1A levels are associated with insulin resistance. However, how PGC1A activity impacts the hepatic insulin-signaling pathway is still unclear. We used gain- and loss-of-function models in mouse primary hepatocytes and measured hepatocyte insulin response by gene and protein expression and ex vivo glucose production. We found that the PGC1A level determines the relative ratio of IRS1 and IRS2 in hepatocytes, impacting insulin receptor signaling via protein kinase B/AKT (AKT). PGC1A drove the expression of IRS2 downstream of glucagon signaling while simultaneously reducing IRS1 expression. We illustrate that glucagon- or PGC1A-induced IRS2 expression was dependent on cAMP Response Element Binding Protein activity and that this was essential for suppression of hepatocyte gluconeogenesis in response to insulin in vitro. We also show that increased hepatic PGC1A improves glucose homeostasis in vivo, revealing a counterregulatory role for PGC1A in repressing uncontrolled glucose production in response to insulin signaling. These data highlight a mechanism by which PGC1A plays dual roles in the control of gluconeogenesis during the fasting-to-fed transition through regulated balance between IRS1 and IRS2 expression.
Keywords: PGC-1; Ppargc1a; fasting; gluconeogenesis; liver.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling.Cell Metab. 2005 Nov;2(5):331-8. doi: 10.1016/j.cmet.2005.09.008. Cell Metab. 2005. PMID: 16271533
-
PHD3 regulates glucose metabolism by suppressing stress-induced signalling and optimising gluconeogenesis and insulin signalling in hepatocytes.Sci Rep. 2018 Sep 24;8(1):14290. doi: 10.1038/s41598-018-32575-z. Sci Rep. 2018. PMID: 30250231 Free PMC article.
-
Gluconeogenic signals regulate iron homeostasis via hepcidin in mice.Gastroenterology. 2014 Apr;146(4):1060-9. doi: 10.1053/j.gastro.2013.12.016. Epub 2013 Dec 17. Gastroenterology. 2014. PMID: 24361124 Free PMC article.
-
Divergent Roles of IRS (Insulin Receptor Substrate) 1 and 2 in Liver and Skeletal Muscle.Curr Med Chem. 2017;24(17):1827-1852. doi: 10.2174/0929867324666170426142826. Curr Med Chem. 2017. PMID: 28462703 Review.
-
IRS2 and PTP1B: Two opposite modulators of hepatic insulin signalling.Arch Physiol Biochem. 2011 Jul;117(3):105-15. doi: 10.3109/13813455.2011.557386. Epub 2011 Mar 14. Arch Physiol Biochem. 2011. PMID: 21401320 Review.
Cited by
-
Transcriptome-wide analysis of PGC-1α-binding RNAs identifies genes linked to glucagon metabolic action.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22204-22213. doi: 10.1073/pnas.2000643117. Epub 2020 Aug 26. Proc Natl Acad Sci U S A. 2020. PMID: 32848060 Free PMC article.
-
The Insulin Receptor Adaptor IRS2 is an APC/C Substrate That Promotes Cell Cycle Protein Expression and a Robust Spindle Assembly Checkpoint.Mol Cell Proteomics. 2020 Sep;19(9):1450-1467. doi: 10.1074/mcp.RA120.002069. Epub 2020 Jun 18. Mol Cell Proteomics. 2020. PMID: 32554797 Free PMC article.
-
Calorie restriction and life-extending mutation downregulate miR-34a to facilitate lipid metabolism in the liver.Exp Gerontol. 2024 Sep;194:112506. doi: 10.1016/j.exger.2024.112506. Epub 2024 Jul 10. Exp Gerontol. 2024. PMID: 38945410 Free PMC article.
-
Berberine attenuates fructose-induced insulin resistance by stimulating the hepatic LKB1/AMPK/PGC1α pathway in mice.Pharm Biol. 2020 Dec;58(1):385-392. doi: 10.1080/13880209.2020.1756349. Pharm Biol. 2020. PMID: 32393087 Free PMC article.
-
Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance.Int J Mol Sci. 2021 Jan 9;22(2):623. doi: 10.3390/ijms22020623. Int J Mol Sci. 2021. PMID: 33435513 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

