Defining a murine ovarian cancer model for the evaluation of conditionally-replicative adenovirus (CRAd) virotherapy agents
- PMID: 30767772
- PMCID: PMC6376676
- DOI: 10.1186/s13048-019-0493-5
Defining a murine ovarian cancer model for the evaluation of conditionally-replicative adenovirus (CRAd) virotherapy agents
Abstract
Background: Virotherapy represents a promising approach for ovarian cancer. In this regard, conditionally replicative adenovirus (CRAd) has been translated to the context of human clinical trials. Advanced design of CRAds has sought to exploit their capacity to induce anti-tumor immunization by configuring immunoregulatory molecule within the CRAd genome. Unfortunately, employed murine xenograft models do not allow full analysis of the immunologic activity linked to CRAd replication.
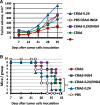
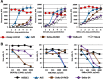
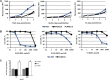
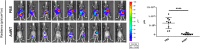
Results: We developed CRAds based on the Ad5/3-Delta24 design encoding cytokines. Whereas the encoded cytokines did not impact adversely CRAd-induced oncolysis in vitro, no gain in anti-tumor activity was noted in immune-incompetent murine models with human ovarian cancer xenografts. On this basis, we explored the potential utility of the murine syngeneic immunocompetent ID8 ovarian cancer model. Of note, the ID8 murine ovarian cancer cell lines exhibited CRAd-mediated cytolysis. The use of this model now enables the rational design of oncolytic agents to achieve anti-tumor immunotherapy.
Conclusions: Limits of widely employed murine xenograft models of ovarian cancer limit their utility for design and study of armed CRAd virotherapy agents. The ID8 model exhibited CRAd-induced oncolysis. This feature predicate its potential utility for the study of CRAd-based virotherapy agents.
Keywords: Adenovirus; Anti-tumor immunization; CRAd; ID8; Ovarian cancer; Virotherapy.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial and non-financial competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Combinatorial strategies based on CRAd-IL24 and CRAd-ING4 virotherapy with anti-angiogenesis treatment for ovarian cancer.J Ovarian Res. 2016 Jun 27;9(1):38. doi: 10.1186/s13048-016-0248-5. J Ovarian Res. 2016. PMID: 27349517 Free PMC article.
-
Treatment of chemotherapy resistant ovarian cancer with a MDR1 targeted oncolytic adenovirus.Gynecol Oncol. 2011 Oct;123(1):138-46. doi: 10.1016/j.ygyno.2011.06.007. Epub 2011 Jul 13. Gynecol Oncol. 2011. PMID: 21741695
-
A fiber-modified mesothelin promoter-based conditionally replicating adenovirus for treatment of ovarian cancer.Clin Cancer Res. 2008 Jun 1;14(11):3582-8. doi: 10.1158/1078-0432.CCR-07-5053. Clin Cancer Res. 2008. PMID: 18519792
-
Armed replicating adenoviruses for cancer virotherapy.Cancer Gene Ther. 2009 Jun;16(6):473-88. doi: 10.1038/cgt.2009.3. Epub 2009 Feb 6. Cancer Gene Ther. 2009. PMID: 19197323 Free PMC article. Review.
-
Concepts in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2021 Sep 29;22(19):10522. doi: 10.3390/ijms221910522. Int J Mol Sci. 2021. PMID: 34638863 Free PMC article. Review.
Cited by
-
SOCS3 inhibiting JAK-STAT pathway enhances oncolytic adenovirus efficacy by potentiating viral replication and T-cell activation.Cancer Gene Ther. 2024 Mar;31(3):397-409. doi: 10.1038/s41417-023-00710-2. Epub 2023 Dec 15. Cancer Gene Ther. 2024. PMID: 38102464
-
Targeting CA-125 Transcription by Development of a Conditionally Replicative Adenovirus for Ovarian Cancer Treatment.Cancers (Basel). 2021 Aug 24;13(17):4265. doi: 10.3390/cancers13174265. Cancers (Basel). 2021. PMID: 34503075 Free PMC article.
-
In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer.Int J Mol Sci. 2023 Jun 10;24(12):9992. doi: 10.3390/ijms24129992. Int J Mol Sci. 2023. PMID: 37373140 Free PMC article.
-
Influences of Gastrointestinal Microbiota Dysbiosis on Serum Proinflammatory Markers in Epithelial Ovarian Cancer Development and Progression.Cancers (Basel). 2022 Jun 20;14(12):3022. doi: 10.3390/cancers14123022. Cancers (Basel). 2022. PMID: 35740687 Free PMC article.
-
Understanding and addressing barriers to successful adenovirus-based virotherapy for ovarian cancer.Cancer Gene Ther. 2021 May;28(5):375-389. doi: 10.1038/s41417-020-00227-y. Epub 2020 Sep 19. Cancer Gene Ther. 2021. PMID: 32951021 Free PMC article. Review.
References
-
- Park JW, Kim M. Replicating viruses for gynecologic cancer therapy. Eur J Gynaecol Oncol. 2016;37(3):295–304. - PubMed
-
- Hartkopf AD, Fehm T, Wallwiener D, Lauer U. Oncolytic virotherapy of gynecologic malignancies. Gynecol Oncol. 2011;120(2):302–310. - PubMed
-
- Matthews KS, Alvarez RD, Curiel DT. Advancements in adenoviral based virotherapy for ovarian cancer. Adv Drug Deliv Rev. 2009;61(10):836–841. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

