Novel Insights reveal Anti-microbial Gene Regulation of Piglet Intestine Immune in response to Clostridium perfringens Infection
- PMID: 30760749
- PMCID: PMC6374412
- DOI: 10.1038/s41598-018-37898-5
Novel Insights reveal Anti-microbial Gene Regulation of Piglet Intestine Immune in response to Clostridium perfringens Infection
Abstract
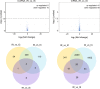
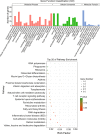
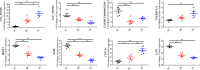
LncRNA play important roles in regulation of host immune and inflammation responses in defending bacterial infection. Clostridium perfringens (C. perfringens) type C is one of primary bacteria leading to piglet diarrhea and other intestinal inflammatory diseases. For the differences of host immune capacity, individuals usually show resistance and susceptibility to bacterial infection. However, whether and how lncRNAs involved in modulating host immune resistance have not been reported. We have investigated the expression patterns of ileum lncRNAs of 7-day-old piglets infected by C. perfringens type C through RNA sequencing. A total of 16 lncRNAs and 126 mRNAs were significantly differentially expressed in resistance (IR) and susceptibility (IS) groups. Many lncRNAs and mRNAs were identified to regulate resistance and susceptibility of piglets through immune related pathways. Five lncRNAs may have potential function on regulating the expressions of cytokines, these lncRNAs and cytokines work together to co-regulated piglet immune response to C. perfringens, affecting host resistance and susceptibility. These results provide valuable information for understanding the functions of lncRNA and mRNA in affecting piglet diarrhea resistance of defensing to C. perfringens type C, these lncRNAs and mRNAs may be used as the important biomarkers for decreasing C. perfringens spread and diseases in human and piglets.
Conflict of interest statement
The authors declare no competing interests.
Figures
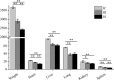

Similar articles
-
Integrative Analyses of Long Non-coding RNA and mRNA Involved in Piglet Ileum Immune Response to Clostridium perfringens Type C Infection.Front Cell Infect Microbiol. 2019 Apr 30;9:130. doi: 10.3389/fcimb.2019.00130. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31114763 Free PMC article.
-
Effect of Clostridium perfringens type C on TLR4/MyD88/NF-κB signaling pathway in piglet small intestines.Microb Pathog. 2019 Oct;135:103567. doi: 10.1016/j.micpath.2019.103567. Epub 2019 Jun 1. Microb Pathog. 2019. PMID: 31163250
-
Identification and Characterization of MAPK Signaling Pathway Genes and Associated lncRNAs in the Ileum of Piglets Infected by Clostridium perfringens Type C.Biomed Res Int. 2020 Aug 12;2020:8496872. doi: 10.1155/2020/8496872. eCollection 2020. Biomed Res Int. 2020. PMID: 32855971 Free PMC article.
-
Clostridial diarrheas in piglets: A review.Vet Microbiol. 2023 May;280:109691. doi: 10.1016/j.vetmic.2023.109691. Epub 2023 Feb 23. Vet Microbiol. 2023. PMID: 36870204 Review.
-
Clostridial enteric infections in pigs.J Vet Diagn Invest. 2005 Nov;17(6):528-36. doi: 10.1177/104063870501700602. J Vet Diagn Invest. 2005. PMID: 16475510 Review.
Cited by
-
Decreased S100A9 expression alleviates Clostridium perfringens beta2 toxin-induced inflammatory injury in IPEC-J2 cells.PeerJ. 2023 Jan 25;11:e14722. doi: 10.7717/peerj.14722. eCollection 2023. PeerJ. 2023. PMID: 36718447 Free PMC article.
-
lnc001776 Affects CPB2 Toxin-Induced Excessive Injury of Porcine Intestinal Epithelial Cells via Activating JNK/NF-kB Pathway through ssc-let-7i-5p/IL-6 Axis.Cells. 2023 Mar 29;12(7):1036. doi: 10.3390/cells12071036. Cells. 2023. PMID: 37048109 Free PMC article.
-
ssc-miR-185 targets cell division cycle 42 and promotes the proliferation of intestinal porcine epithelial cell.Anim Biosci. 2021 May;34(5):801-810. doi: 10.5713/ajas.20.0325. Epub 2020 Oct 12. Anim Biosci. 2021. PMID: 33152231 Free PMC article.
-
Identification of MicroRNAs Regulating Clostridium perfringens Type C Infection in the Spleen of Diarrheic Piglets.Curr Issues Mol Biol. 2023 Apr 6;45(4):3193-3207. doi: 10.3390/cimb45040208. Curr Issues Mol Biol. 2023. PMID: 37185732 Free PMC article.
-
Change in Long Non-Coding RNA Expression Profile Related to the Antagonistic Effect of Clostridium perfringens Type C on Piglet Spleen.Curr Issues Mol Biol. 2023 Mar 9;45(3):2309-2325. doi: 10.3390/cimb45030149. Curr Issues Mol Biol. 2023. PMID: 36975519 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

